In 1974, while screening blood donations for hepatitis B, an Australian virologist accidentally discovered a new parvovirus (parvo meaning “small”). Many other parvoviruses that cause infection in animals were known, but this was the first to be found in humans.
This human parvovirus (hPV) was named B19 because it was found in sample 19 of panel B in the batch of tests. It was not known what disease, if any, it caused until 1981, when it was found to be a cause of “aplastic crisis” – severe, life-threatening anaemia in children.
Then, in 1983, it was shown to be the cause of the common childhood rash-illness, erythema infectiosum (also known as “slapped cheek” syndrome or “fifth disease”). Now that rubella and measles are rare in countries with widespread immunisation, erythema infectiosum is the commonest cause of infectious childhood rash.
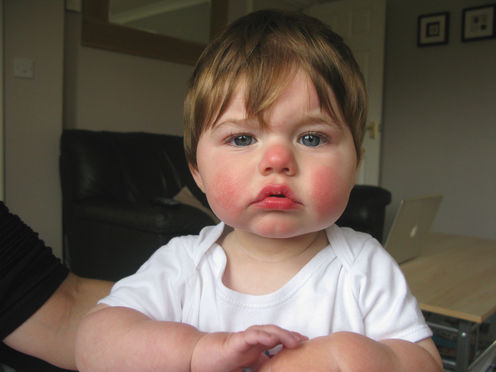
“Slapped cheek” describes the typical bright red rash on the face caused by hPV infection; on the limbs and body the rash typically has a lacy appearance. Other symptoms, if any, are mild and short-lived in most people, but adults, particularly, can have pain in the joints of the hands, wrists and knees.
One in five people will have only vague symptoms, such as mild fever or feeling generally unwell, or none at all. Children who contract the virus are not excluded from childcare, school or work, but are advised to rest at home until they feel better.
How hPV causes disease
Human parvovirus infects the precursors of red blood cells in the bone marrow and temporarily stops them developing. This causes anaemia, which is usually mild, short-lived and insignificant in otherwise healthy people.
As the body’s immune system responds, hPV is removed and red blood cell production resumes normally. The rash and joint pains are due to the body’s immune response.
People whose immune systems are suppressed for any reason can have prolonged, chronic infection with severe anaemia, but no rash. Other very rare complications have been reported, including include encephalitis (inflammation of the brain) and various blood and autoimmune diseases, but their overall frequency and significance are unknown.
The virus spreads by droplets in our breath or, rarely, by transfusion of blood. It can also spread from a pregnant woman to her foetus. Typically, a week or two after contact with an infected person, there are some vague symptoms such as mild fever and malaise and the rash appears a few days later.
Outbreaks of hPV infection occur in prolonged epidemics lasting many months, usually in late winter and early spring. Epidemics mainly occur among primary-school-aged children and usually last for a year or more. Then there are few if any cases for two to four years, then another epidemic starts.
During outbreaks about 50% of susceptible children (those who haven’t had it before and thus aren’t immune) in affected schools and their household contacts will be infected. The rate of infection in other susceptible contacts, such as schoolteachers, is about 20%.
The proportion of people with hPV antibodies in their blood, indicating past infection and immunity, increases with age from about 15% in preschool children, to 50% in young adults and 85% in the elderly.
Pregnancy and hPV infection
When a pregnant woman is infected with hPV, the likelihood that her foetus will be infected is about 50%. Most will have no significant ill-effect – the only evidence that the foetus has been infected is hPV antibodies in the baby’s blood. However, when the mother’s infection is in the first trimester, there is an increased risk of miscarriage (about 10%).
Later in the first half of pregnancy, between nine and 20 weeks, foetal infection can cause severe anaemia (about 3% risk). Foetuses have a high turnover of red blood cells. When new red cell development is interrupted by infection of their precursors, production cannot keep up with demand.
Severe foetal anaemia can cause heart failure, which in turn causes swelling throughout the body – a potentially fatal condition known as hydrops fetalis.
Hydrops usually develops five to eight weeks after the mother’s infection. It causes foetal death in up to a third of cases. A similar proportion of cases recover spontaneously. If detected in time, hydrops often can be treated successfully by blood transfusion into the uterus.
In skilled hands this is a safe procedure, which has been used to treat foetal hydrops for many years. Because timing is critical, it is important to confirm the mother’s hPV infection as soon as possible after it is suspected. This is done by testing her blood for a type of hPV antibody that is detectable for only a few weeks (immunoglobulin M), or by showing an increase in the level of immunoglobulin G, which persists indefinitely after infection.
Once the mother’s infection has been confirmed, frequent ultrasound examinations over the next few months are recommended, to detect the rare cases of hydrops. If it occurs, the mother should be referred to an experienced foetal medicine specialist.
There is no specific treatment or vaccine for hPV infection. Fortunately, it is nearly always mild or asymptomatic, but we need to know more about the very rare, but potentially serious, complications.