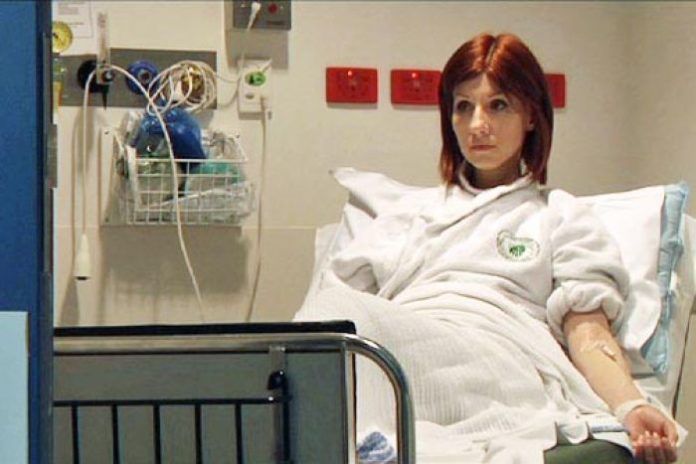
At the age of 36, Danielle Tindle has been given an ultimatum — your money or your life.
A decade after surviving Hodgkin’s lymphoma, Dr Tindle is critically ill with another rare cancer.
But this time, she is being forced to pay thousands of dollars for potentially life-saving drugs which are available at minimal cost to other patients under the Pharmaceutical Benefits Scheme (PBS).
When Dr Tindle gets her immunotherapy for cancer every two weeks, it costs her nearly $5,000 a shot.
In the private hospital cubicle next to her could be a melanoma patient who is paying just $6.20 per treatment.
The discrepancy is due to drug listing requirements for the PBS.
“[The melanoma patient] is receiving it at a PBS subsidised rate, while I’m paying thousands of dollars,” Dr Tindle told Australian Story.
“There’s no-one that could look me in the eye, from any level of government, or even the drug companies, and say that’s a fair situation. Something needs to change.”
Rare Cancers Australia chief executive Richard Vines said: “There’s a lot of people out there who are very surprised when they get diagnosed with a rare cancer, that what they thought was a good, safe health system which provided drugs for people, just doesn’t.
“Nobody thinks it’s fair. It’s so frustrating.
“The [drugs] are sitting on the shelf, the Government’s OK’d them for one type of cancer, and not for the other.”

Australian Story featured Dr Tindle in 2005, after she survived Hodgkin’s lymphoma.
Ultimately her life was saved by her father’s own groundbreaking stem-cell research.
Since then, she has advocated for improved services and care for other young cancer patients, and become a world leader in the field.
“Danielle is someone who has fought really hard to make a difference to people like me, and to future adolescent and young adult patients,” said Jasmine Gailer, a former cancer patient and founder of cancer support charity, Scar Stories.
New cancer caused by toxic cancer treatment, father says
Midway through her PhD, entitled Creating Meaning: The cancer survivorship experiences of young adults in Australia, England and the United States, Dr Tindle was told she had cancer again.
“It wasn’t a relapse of her Hodgkin’s lymphoma, it was a new tumour altogether — a neuroendocrine carcinoma,” Professor Robert Tindle, Danielle’s father, said.
“That she’s got it is almost entirely the result of the draconian treatment she had 12 years ago for Hodgkin’s lymphoma,” the retired Professor in Immunology believes.
Some doctors support this theory that Dr Tindle’s new cancer may have been caused by the toxic treatment she received in her early 20s.
“Certainly chemotherapy can cause genetic alterations and there is that chance of developing other tumours. But generally, neuroendocrine tumours are extremely rare,” Dr Margie McGrath, medical oncologist at Greenslopes Private Hospital in Brisbane, said.
“So whether Danielle’s just very unlucky, it’s hard to say.”
All conventional therapies have failed in Dr Tindle’s case.

Her father, in conjunction with the Peter MacCallum Cancer Centre in Melbourne, sent her tumour tissue for genomic profiling to the US.
The results of that profile showed her tumour was very similar to small-cell carcinoma.
Early results from trials of the immunotherapy drug nivolumab in combination with ipilimumab in small cell lung cancer have shown promising response rates.
Some doctors see these drugs as potentially the last option in the attempt to prolong Dr Tindle’s life.
“These trials provided the most relevant information upon which to consider ongoing treatment options for Danielle when standard treatments had failed,” Dr McGrath said.
There have been no phase three clinical trials into the drugs in relation to neuroendocrine cancer, and therefore the Therapeutic Goods Administration has not approved them for use in that condition, and they have not been listed on the PBS.

“By law, for the PBAC to make a recommendation to the Minister for listing of medicines on the PBS, it must consider the evidence of the medicine’s relative effectiveness and cost-effectiveness,” a spokeswoman for the Federal Department of Health said in a statement to the ABC.
“It does this on the basis of applications from the sponsoring company. The sponsor of nivolumab and ipilimumab has not applied to list this drug or a combination of drugs for this condition on the PBS.”
Mr Vines argued: “We’ve had great breakthroughs in producing really clever drugs that work in many different cancers.
“We need to change the Pharmaceutical Benefits Advisory Committee and PBS thinking, so that they look at the mechanism of cancer, not the location of it.”
Dr Tindle will have scans at the end of May to see whether or not the new medicines have been effective in shrinking her tumour.
So far she has paid nearly $80,000 for immunotherapy drugs since July last year.
“This is happening to a … number of people around Australia,” Dr Tindle said.
“I can’t help thinking I have given so much, a lot of my advocacy work has been pro bono work, but when I need help, I feel like the system is failing me.”