Researchers have identified a mechanism that allows cancer cells to respond and grow rapidly when levels of sugar in the blood rise. This may help to explain why people who develop conditions in which they have chronically high sugar levels in their blood, such as obesity, also have an increased risk of developing certain types of cancer.
The findings were published in the journal eLife, by Susumu Hirabayashi who leads the Metabolism and Cell Growth group at the MRC Clinical Sciences Centre based at Imperial College London, and Ross Cagan of the Icahn School of Medicine at Mount Sinai, in New York.
When we eat food it’s digested into smaller molecules, such as glucose, that pass into our blood stream. As blood is pumped around the body, it delivers glucose to the body’s cells, which use it as fuel to grow. For glucose to be absorbed efficiently, it teams up with a hormone called insulin. Insulin acts like a gatekeeper: it binds to receptors on the cell’s surface and triggers a ‘gate’, or channel, to open and let glucose in.
People with obesity often have persistently high levels of glucose and insulin in the blood. Over time this fades to background noise and the body tunes out, or becomes ‘insulin resistant’. With the gate closed, glucose can’t be absorbed efficiently so it builds up in the blood and this accumulation can ultimately lead to type 2 diabetes.
But not all cells tune out. In fact, Hirabayashi and colleagues have previously shown that tumour cells in the fruit fly Drosophila melanogaster actively tune in. In a study published two years ago, the team engineered flies to activate the genes ‘Ras’ and ‘Src’, which are activated in a variety of cancers in people. Flies share many of the same genes that control metabolism and cell growth in people, so the findings from the study may be relevant to us too.
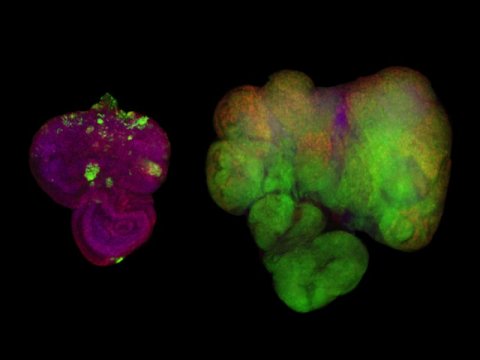
The scientists activated Ras and Src in the flies’ developing eye tissue. They also labelled these cells so that they would glow green under fluorescent light. Flies that were fed a normal diet grew small, benign tumours (pictured, left). But when these flies were fed a high-sugar diet, they developed large, malignant tumours (pictured, right).
Hirabayashi found that in flies fed a high-sugar diet, the ‘normal’ cells became insulin resistant, but the tumour cells didn’t. The tumour cells actually became more sensitive to insulin because they turned on a metabolic switch that triggered them to produce extra receptors for insulin. With insulin binding to many more receptors than usual, more glucose channels opened up and the tumour cells became a ‘sink’ for the glucose that had nowhere else to go in the insulin resistant body of the fly. But this earlier study did not explain how the tumour cells turned on this metabolic switch.
Hirabayashi and Cagan studied the same flies in more detail, and in this latest study they show that the tumour cells detect glucose availability indirectly, through a protein called Salt-inducible kinase (SIK). When glucose levels are high, SIK sends a signal along a route called the ‘Hippo signalling pathway’.
The Hippo signalling pathway is known to play a role in controlling cell growth. When it’s turned on it keeps cell growth under control, but if it is turned off, the cell can carry on growing, and may ultimately develop into a tumour. Hirabayashi and Cagan found that SIK acts like a sugar sensor, turning the Hippo signalling pathway off, in response to raised glucose levels. This allows the tumour cells to continue to grow.
“Ras and Src co-activated tumours use SIK to sense that there’s lots of glucose available outside of their cells, and to tell the cells to take advantage of that,” says Hirabayashi. These tumours are extremely sensitive and poised to respond quickly to changes in the Hippo signalling pathway. “Together, Ras and Src co-activated tumours use SIK to efficiently respond to glucose availability and ensure the tumours grow in nutrient rich condition such as obesity. We still don’t know if tumours caused by other genes respond to sugar in the same way.”
The findings are relevant to people with obesity who often have chronically raised sugar levels. “Our results suggest that if we can develop drugs to target SIK, and stop it from alerting cancer cells in this way, then we may be able to stop cancer cells from thriving in an insulin-resistant environment, and break the connection between obesity and cancer,” says Hirabayashi.
Before scientists can develop drugs to block SIK, they must first confirm that a similar mechanism happens in people.
Conditions such as obesity and cancer are too often studied in isolation, Hirabayashi, says. “If researchers from these two fields of expertise were to work more closely together, each might gain useful insights from the other about possible connections.”
Story Source:
The above post is reprinted from materials provided by MRC Clinical Sciences Centre/Institute of Clinical Sciences (ICS) Faculty of Medicine, Imperial Coll. The original item was written by Deborah Oakley.Note: Materials may be edited for content and length.
Journal Reference:
- Susumu Hirabayashi, Ross L Cagan. Salt-inducible kinases mediate nutrient-sensing to link dietary sugar and tumorigenesis inDrosophila. eLife, 2015; 4 DOI: 10.7554/eLife.08501