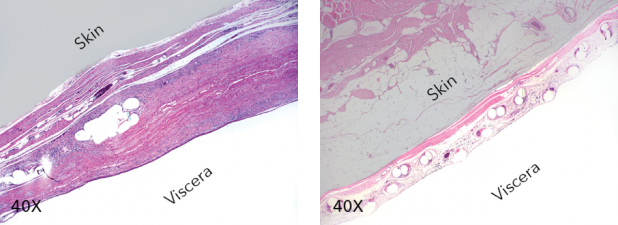
Left: Zenapro histologic cross-section demonstrating increased tissue deposition and incorporation. Right: Hydrogel-coated synthetic mesh histologic cross-section
 At a recent SAGES Advanced Hernia Course in Indianapolis, Indiana, Cook Medical demonstrated Zenapro, a next-generation hybrid hernia repair technology that combines outer layers of extracellular matrix with a polypropylene mesh. The polypropylene mesh is vacuum pressed and laminated between several layers of small intestinal submucosa. The hybrid mesh is then sterilized and sealed for clinical use. In adhesiogenesis models, the extracellular matrix outer layers have demonstrated comparatively low levels of inflammatory response promoting in-growth and lasting durability necessary for complex hernia repairs.
At a recent SAGES Advanced Hernia Course in Indianapolis, Indiana, Cook Medical demonstrated Zenapro, a next-generation hybrid hernia repair technology that combines outer layers of extracellular matrix with a polypropylene mesh. The polypropylene mesh is vacuum pressed and laminated between several layers of small intestinal submucosa. The hybrid mesh is then sterilized and sealed for clinical use. In adhesiogenesis models, the extracellular matrix outer layers have demonstrated comparatively low levels of inflammatory response promoting in-growth and lasting durability necessary for complex hernia repairs.
The technology is already FDA cleared for use in repairing hernias requiring the addition of a reinforcing or bridging material. The hybrid mesh is available in various sizes to accommodate various defects. The technology is being further evaluated in a post-market observational study in 63 patients and 5 centers with results expected in May 2016.
Product page: Zenapro…
The post Cook Medical’s Zenapro Hybrid Hernia Repair Technology appeared first on Medgadget.