For the past year and a half, the Ebola virus has affected thousands, claiming over 11,000 lives to date. The disease is now considered contained, and Liberia has been free of cases for seven weeks, until a new case emerged this morning. The need to employ constant vigilance and surveillance remains high.
For the past year and a half, the Ebola virus has affected thousands, claiming over 11,000 lives to date. The disease is now considered contained, and Liberia has been free of cases for seven weeks, until a new case emerged this morning. The need to employ constant vigilance and surveillance remains high.
Testing for the virus is mostly done through nucleic acid testing, which requires highly trained personnel and expensive equipment and resources. The WHO has reviewed the CorGENIX ReEBOV Antigen Rapid Test Kit, a point-of-care screening test to rapidly check a patient’s blood for presence of the virus. The review followed the Emergency Assessment and Use procedure, which sets the minimum quality, safety, and performance requirements for diagnostic devices for the Ebola emergency. As such, the product is listed only for procurement, and not prequalified yet.
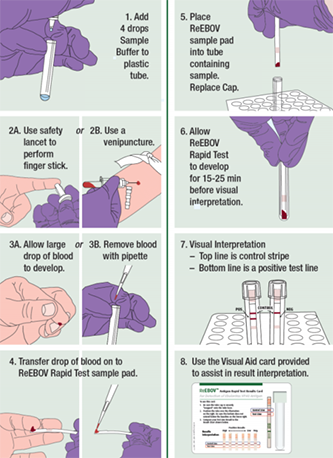
The CorGENIX ReEBOV Antigen Rapid Test Kit is a dipstick that works by capturing EBOV VP40 antigen present in a sample of patient blood. The dipstick is striped with EBOV VP40-specific antibodies conjugated to gold nanoparticles, which, in the presence of antigen, form a complex that’s carried to the reagent site, and forms a pink line that correlates to the concentration of EBOV VP40 antigen in the sample. You can imagine it as being similar to how a pregnancy test works, but using blood instead of urine. In their report in The Lancet, the researchers compared the ReEBOV fingerstick testing to RT-PCR (a nucleic acid test, used as a reference) in 106 patients, and found a sensitivity and specificity of 100% and 92%, respectively.
CorGENIX will continue to conduct post-market surveillance of the safety and efficacy of its test, and the WHO will also be monitoring its performance in the field.
WHO: First Antigen Rapid Test for Ebola through Emergency Assessment and Eligible for Procurement…
Link: ReEBOV Antigen Rapid Test fact sheet…
The post CorGENIX ReEBOV Antigen Rapid Test Kit approved by WHO for emergencies appeared first on Medgadget.