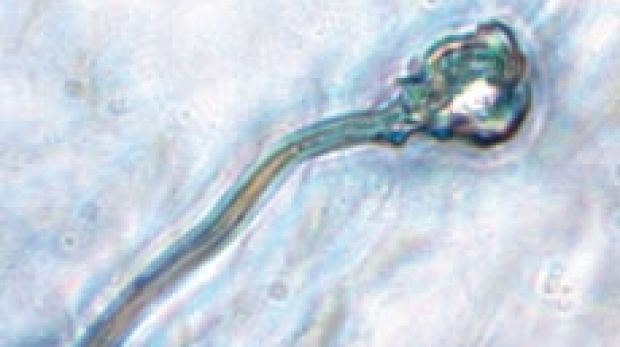
Sperm created from embryonic stem cells at Newcastle University in Britain. Photo: Supplied
London: Human sperm cells have been grown in a laboratory for the first time, in a breakthrough that could lead to a treatment for male infertility, scientists claim.
A French firm said it had produced “fully formed” sperm from basic reproduction cells.
The research has not yet been published in a peer-reviewed journal, and experts greeted the news with caution. However, if proven, the technique could offer hope to people who cannot have children.
The Kallistem laboratory in Lyon said its finding paved the way for new methods of treating infertile men.
“At the end of 2014, the company was able to produce fully formed human spermatozoa [sperm cells] in the laboratory setting, using patient testicular biopsies containing only immature germ cells, or spermatogonia,” the company said in a statement.
“This research paves the way for innovative therapies to preserve and restore male fertility, a major issue with global impact; numbers of spermatozoa have declined by 50 per cent over the last 50 years.”
Joyce Harper, professor of human genetics and embryology at University College London, said: “This is a great move forward – it is really exciting.”
However, she added: “They have said that this is still early stages and we’ve got a long way to go, and that is absolutely correct. There are a number of studies that need to be done.
“The only way you can check if a sperm is really viable is if it fertilises an egg and goes on and develops into a baby.”
Professor Harper said most infertile men did produce sperm that can be extracted from their reproductive system as part of specialised treatment.
Kallistem’s new treatment would be for those who do not produce any mature sperm at all, she added, although, in these cases, the resulting cells would have to be checked to ensure they are “genetically normal”.
Spermatogenesis, the process through which basic reproduction cells – the germ cells – develop into sperm cells, is an extremely complex one. It takes about 72 days in the human body. The scientists said their research could help tens of thousands of men.
Infertility affects at least 10 per cent of couples, and in at least a third of cases it relates to male fertility problems, which are often genetic.
The most common defect is missing regions of male Y chromosomes, which is associated with the production of few or no sperm.
Under the new process, experts would be able to extract reproductive cells from a man’s testes and then freeze them until he wishes to father a child, the firm said.
However, Professor Allan Pacey, of the University of Sheffield, urged couples to treat the announcement with caution.
He said: “This is a bold claim to make and we have had our fingers burnt before. Until I see a peer-reviewed scientific publication showing unequivocally that this has been done, I have to remain sceptical.
“Claims like this can often cause heartache for infertile couples who see them as hope only to have their hopes dashed later when it doesn’t translate into an available procedure.”
Kallistem, which is trying to raise funding for its research, will hold preclinical trials until next year and clinical trials in 2017.
“If it works, this procedure opens great prospects,” said Nathalie Rives, the manager of a fertility clinic.
However, like Professor Harper, she warned that adults suffering from a complete lack of sperm might have “genetic anomalies” that could exclude them from the process.
Professor Israel Nisand, co-founder of the European Bioethics Forum, said the procedure was preferable to reproductive cloning.
Previously, lab-created spermatogenesis had been successful only in mice.
Last year, American researchers announced that they had used skin from infertile men to create sperm after the samples were implanted into the testes of mice.
The samples were genetically engineered to assume the properties of embryonic stem cells beforehand.
Telegraph, London