4D implant saves three babies with respiratory ailments

Three baby boys with life-threatening breathing problems are alive today thanks to a 4D biomaterial, a medical implant designed to change shape over time, that helped them keep breathing, researchers say.
“Today, we see a way to cure a disease that has been killing children for generations,” said Dr. Glenn Green, a pediatric otolaryngologist at the University of Michigan’s C.S. Mott Children’s Hospital and the senior author of a new report on the boys’ cases.
The researchers said that 4D biomaterials could one day help not only patients with respiratory ailments, but also those with disorders involving the heart, bones, muscles or gut.
“The possibilities are really limitless,” lead study author Dr. Robert Morrison, a research fellow and resident surgeon at the University of Michigan Health System, told Live Science.
The researchers made the implants using a 3D printer. Three-dimensional printers can create items from a wide variety of materials: plastic, ceramic, glass, metal and even more unusual ingredients such as living cells. The machines work by depositing layers of material, just as ordinary printers lay down ink, except 3D printers can also lay down flat layers on top of each other to build 3D objects.
Advances in 3D printing have enabled the rapid production of medical devices that are customized for individual patients, such as hearing aids, dental implants and prosthetic hands. However, devices made of rigid materials are often unsuitable for young patients who can quickly outgrow the implants.
Recently, scientists began developing techniques to try to accomplish 4D printing, which involves 3D printing items that are designed to shape-shift after they are printed. Green and his colleagues reasoned that 4D items could grow with young patients if needed.
“This is the first 3D-printed implant specifically designed to change shape over time, the fourth dimension, to allow for a child’s growth,” Green told Live Science.

Image: Morrison et al., Science Translational Medicine (2015)
The three infant boys who were implanted with the new device all had the same life-threatening condition — a severe form of a disease called tracheobronchomalacia, which affects about 1 in 2,000 children around the world. The disease causes the windpipe to regularly collapse, preventing normal breathing. There was no cure, and at the time these children received their implants, their life expectancies were estimated at days to weeks, Green said.
“It is hard to convey how very sick these children were,” Green said. All three boys had been in the intensive care unit for months. During that time, to stay alive, they often needed drugs to keep them sedated and prevent them from moving. They all had breathing tubes placed in their necks, and were on artificial ventilators — but still, they sufferedrepeatedly suffered of breathing problems and needed to be resuscitated.
One boy, Kaiba Gionfriddo, was 3 months old when the doctors implanted the new device. Gionfriddo had turned blue when he was a newborn because his lungs were not getting the oxygen they needed. Five-month-old Ian Orbich was unable to have any food in his stomach without suffering cardiac arrests.
Sixteen-month-old Garrett Peterson’s airways “were floppy, with the consistency of a wet noodle,” Garrett’s mother Natalie Peterson told Live Science. “They collapsed pretty much daily, if not multiple times a day. Simple things like changing his diaper or holding him could cause Garrett’s airways to collapse. This happened repeatedly for months.”
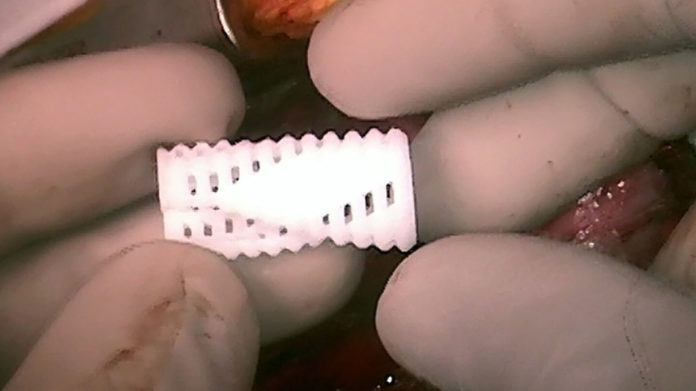
The researchers used CT scans of the infants to develop 3D-printed airway splints whose length, diameter, thickness and other factors were customized for each baby, to help keep the infants’ weakened airways open.

Image: Morrison et al., Science Translational Medicine (2015)
“We can print tens or hundreds of the exact same splint design, no matter how complicated the geometry is,” study co-author Scott Hollister, a biomedical engineer at the University of Michigan, told Live Science. “This is very important for quality and design control, because we can take copies or replicas of the exact same splint and test it before we even implant it.”
The splints were shaped a bit like hot dog buns, and when they were implanted into the infants and, sewn around their own windpipes, the devices kept surrounding tissue from pushing in and sealing the airways shut. The devicessplints are made of a material called, polycaprolactone, which harmlessly dissolves in the body over time.
“This is the first time 3D printing has been used to create a medical implant for treating a life-threatening disease,” Morrison said.
The airway splints were hollow and porous, designed to spread open as the children grew. Green noted that the infants’ bronchi, which are the air passages connecting the windpipe with the lungs, were about the width of a pencil lead when the devices were implanted, but will nearly double in diameter by the time the splints dissolve.
The researchers followed the growth of the airways over time with CT and MRI scans. They found the devices improved breathing and expanded to allow the airways to grow in all three patients. All the devices are dissolving as expected, and none of the devices have caused any complications.

Kaiba Gionfriddo, who was born with a rare condition that caused life-threatening breathing problems. Above, Kaiba and his mother April.
Image: University of Michigan Health System
The boys are now home with their families. They no longer need sedatives, narcotics or paralytics to keep them breathing. “Holidays are not spent in the hospital anymore,” Green said. “Instead of lying flat on their backs for weeks on end, these children are learning to sit and stand and run.”
“I honestly don’t think we can ever thank Dr. Green and his team in Michigan enough,” Natalie Peterson said. “We know that without this procedure, Garrett was a month or so from passing away.”
If children with tracheobronchomalacia survive to age 2 or 3, their airways usually grow strong enough to overcome the disorder, and their windpipes eventually will have no signs of the disease that nearly killed them as newborns. The researchers say the airway splints will dissolve slowly enough to help the infants reach this point. The first child to receive the device, Kaiba, has now reached this stage, and “he is doing well after degradation of the splint,” Green said.
“Before this procedure, babies with severe tracheobronchomalacia had little chance of surviving,” Green said in a statement. “Today, our first patient, Kaiba, is an active, healthy 3-year-old in preschool with a bright future. The device worked better than we could have ever imagined.”
“The first time he was hospitalized, doctors told us he may not make it out,” Kaiba’s mom, April Gionfriddo, said in a statement. “It was scary knowing he was the first child to ever have this procedure, but it was our only choice, and it saved his life.”
Garrett is now 2-and-a-half years old. “Garrett’s doing awesome now,” the boy’s father, Jake Peterson, told Live Science. “He’s so happy — there’s no hint of an issue with his airway splint. He’s starting to learn to sit up on his own.”
Ian Orbich is now 17 months old, and the researchers say he is known for his grins, enthusiastic high fives and love for playing with his big brother, Owen.
“We were honestly terrified, just hoping that we were making the right decision,” Ian’s mother, Meghan Orbich, said in a statement. “I am thankful every single day that this splint was developed. It has meant our son’s life. I am certain that if we hadn’t had the opportunity to bring Ian to Mott, he would not be here with us today.”
The doctors received emergency clearance from the FDA to perform these procedures as a last resort. The researchers are now pursuing a clinical trial for the 4D biomaterials for patients with less severe forms of tracheobronchomalacia.
“We’ve been meeting with the FDA to have a plan set up to have 30 children as part of a clinical trial,” Green said. “These will be children that have severe tracheobronchomalacia, but not the imminently life-threatening tracheobronchomalacia of these first three children.”
Hollister and Green have filed a patent application related to the device. The scientists detailed their findings online on April 29 in the journal Science Translational Medicine.
- ‘Smart Bullet’ Hits Targets On The Run | Video
- Having Mom in the Car Changes Teen Driver’s Brain
- WWII Supermarine Spitfire Still Flying | Video
- Why Some Lithium-Ion Batteries Explode
This article originally published at LiveScience here