Prostate cancer screening with prostate-specific antigen (PSA) has been controversial. Critics of the test (even the person who discovered PSA is a critic!) argue that patients with prostate cancer are more likely to die with it than from it, rendering any testing to be unnecessary. In addition, the United States Preventative Services Task Force recommends against it, stating that it doesn’t save more lives, and that it adds unnecessary testing and treatments that cause pain, impotence, and incontinence in many who don’t actually need it.
Prostate cancer screening with prostate-specific antigen (PSA) has been controversial. Critics of the test (even the person who discovered PSA is a critic!) argue that patients with prostate cancer are more likely to die with it than from it, rendering any testing to be unnecessary. In addition, the United States Preventative Services Task Force recommends against it, stating that it doesn’t save more lives, and that it adds unnecessary testing and treatments that cause pain, impotence, and incontinence in many who don’t actually need it.
At any rate, there is at least a niche for the development of a more reliable test for prostate cancer to clearly differentiate those who need further treatment from those who don’t. With an estimated 233,000 new cases and 29,480 deaths in 2014 in the United States, prostate cancer represents an important disease. It is the second most commonly-diagnosed cancer in men, following skin cancer.
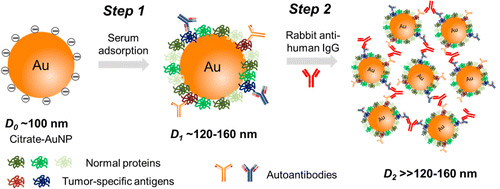
Researchers at the University of Central Florida have devised a $1 screening tool for prostate cancer called NanoDLSay (Nanoparticle-enabled Dynamic Light Scattering Assay). It quantifies the elevated immunoglobulin G (IgG) levels found in prostate cancer blood samples.Specifically, the researchers mixed human serum with citrate-capped gold nanoparticles, which adsorbed IgG. These adsorbed IgG molecules were then connected together with an anti-IgG antibody, effectively creating clumps of gold nanoparticles. By measuring the size of these clumps with dynamic light scattering, the researchers were able to correlate the amount of IgG to the presence of prostate cancer. In a pilot study of 20 prostate cancer samples and 20 control samples, its performance yielded 90-95% sensitivity and 50% specificity. Comparatively, traditional PSA testing has a sensitivity of 21-51%, and a specificity of 91%.
NanoDLSay is being validated for prostate cancer at Florida Hospital, VA Medical Center Orlando, and other hospitals. The researchers founded Nano Discovery Inc. to commercialize it. And because the test evaluates IgG, a common molecule not specific to any kind of tumour, the team is exploring its potential to expand into a general cancer screening tool.
Source: University of Central Florida…
Article from ACS Applied Materials and Interfaces: Gold Nanoparticle-Enabled Blood Test for Early Stage Cancer Detection and Risk Assessment…
The post $1 Nanoparticle Test for Prostate Cancer More Sensitive than PSA appeared first on Medgadget.