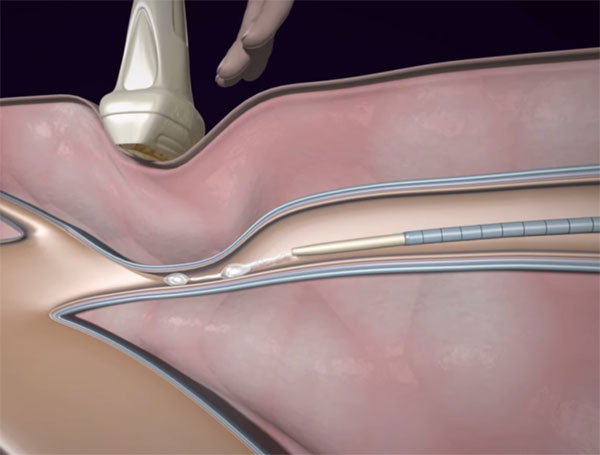
These days varicose veins are typically treated with compression stockings followed by surgery, as necessary. A new product from Sapheon (Morrisville, NC) has just been FDA approved that offers a new approach to closing varicose veins using a minimally invasive approach.
The VenaSeal Sapheon Closure System relies on guiding an intravascular catheter under ultrasound to the treatment site and injecting a medical glue (n-butyl-2-cyanoacrylate) to seal the vein. The technique does not require tumescent anesthesia and the patient is free to go back to normal activities right after treatment.
From the FDA:
The FDA reviewed data for the VenaSeal system in a premarket approval application, the agency’s pathway to evaluate safety and effectiveness of Class III medical devices. Data supporting the FDA approval included results from three clinical studies sponsored by the manufacturer. The U.S. clinical study assessed the safety and effectiveness of the VenaSeal system in 108 participants compared to radio-frequency ablation in 114 participants. The trials showed the device to be safe and effective for vein closure for the treatment of symptomatic superficial varicose veins of the legs.
The VenaSeal system should not be used in patients who have a known hypersensitivity to the VenaSeal adhesive, acute inflammation of the veins due to blood clots or acute whole-body infection. Adverse events observed in the trial—and generally associated with treatments of this condition—included vein inflammation (phlebitis) and burning or tingling (paresthesia) in the treatment zone.
Flashback: VenaSeal Sapheon Vein Closure System Going on Trial in U.S.
Product page: VenaSeal…
Press release: FDA approves closure system to permanently treat varicose veins
The post Sapheon VenaSeal Closure System for Varicose Veins Now FDA Approved (VIDEO) appeared first on Medgadget.