Lack of sleep can elevate levels of free fatty acids in the blood, accompanied by temporary pre-diabetic conditions in healthy young men, according to new research published online February 19, 2015, in Diabetologia, the journal of the European Association for the Study of Diabetes.
The study, the first to examine the impact of sleep loss on 24-hour fatty acid levels in the blood, adds to emerging evidence that insufficient sleep — a highly prevalent condition in modern society — may disrupt fat metabolism and reduce the ability of insulin to regulate blood sugars. It suggests that something as simple as getting enough sleep could help counteract the current epidemics of diabetes and obesity.
“At the population level, multiple studies have reported connections between restricted sleep, weight gain, and type 2 diabetes,” said Esra Tasali, MD, assistant professor of medicine at the University of Chicago and senior author of the study. “Experimental laboratory studies, like ours, help us unravel the mechanisms that may be responsible.”
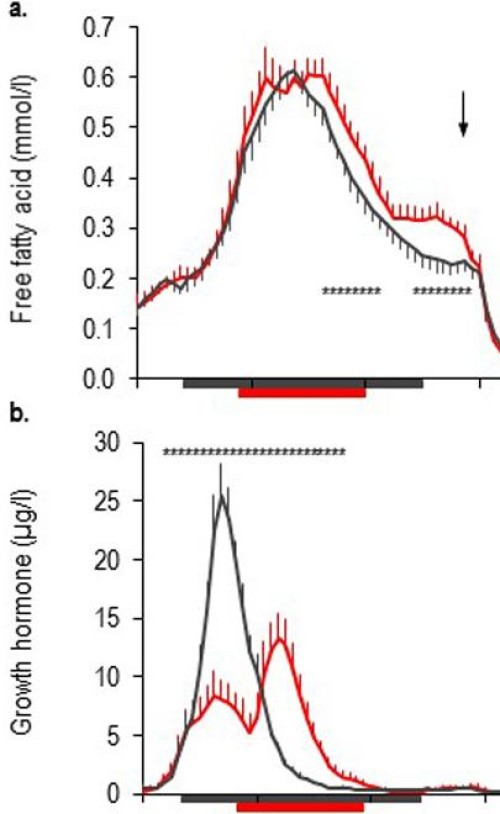
The researchers found that after three nights of getting only four hours of sleep, blood levels of fatty acids, which usually peak and then recede overnight, remained elevated from about 4 a.m. to 9 a.m. As long as fatty acid levels remained high, the ability of insulin to regulate blood sugars was reduced.
The results provide new insights into the connections, first described by University of Chicago researchers 15 years ago, between sleep loss, insulin resistance and heightened risk of type 2 diabetes.
The researchers recruited 19 healthy male subjects between the ages of 18 and 30. These volunteers were monitored through two scenarios in randomized order. In one, they got a full night’s rest — 8.5 hours in bed (averaging 7.8 hours asleep) during four consecutive nights. In the other, they spent just 4.5 hours in bed (averaging 4.3 hours asleep) for four consecutive nights. The two studies were spaced at least four weeks apart.
Each subject’s sleep was carefully monitored, diet was strictly controlled and blood samples were collected at 15 or 30 minute intervals for 24 hours, starting on the evening of the third night of each study. The researchers measured blood levels of free fatty acids and growth hormone, glucose and insulin, and the stress hormones noradrenaline and cortisol. After four nights in each sleep condition, an intravenous glucose-tolerance test was performed.
They found that sleep restriction resulted in a 15 to 30 percent increase in late night and early morning fatty acid levels. The nocturnal elevation of fatty acids (from about 4 a.m. to 6 a.m.) correlated with an increase in insulin resistance — a hallmark of pre-diabetes — that persisted for a nearly five hours.
Cutting back on sleep prolonged nighttime growth hormone secretion and led to an increase in noradrenaline in the blood, both of which contributed to the increase in fatty acid levels.
Although glucose levels were unchanged, the ability of available insulin to regulate blood glucose levels decreased by about 23 percent after a short sleep, “suggesting,” the authors note, “an insulin-resistant state.”
“It definitely looks like a packaged deal,” said the study’s lead author, Josiane Broussard, PhD, a former graduate student at the University of Chicago who is now a post-doctoral research scientist at Cedars-Sinai Medical Center’s Diabetes and Obesity Research Institute in Los Angeles.
“Curtailed sleep produced marked changes in the secretion of growth hormone and levels of noradrenaline — which can increase circulating fatty acids,” Broussard said. “The result was a significant loss of the benefits of insulin. This crucial hormone was less able to do its job. Insulin action in these healthy young men resembled what we typically see in early stages of diabetes.”
Plasma free or non-esterified fatty acids are an important energy source for most body tissues. The demand for fatty acids goes up during exercise, for example, where they are used by cardiac and skeletal muscle; this preserves glucose for use by the brain. But constantly elevated fatty-acid levels in the blood are usually seen only in obese individuals as well as those with type 2 diabetes or cardiovascular disease. A 2012 study by a related research team emphasized the connections between sleep loss and the disruption of human fat cell function in energy regulation.
“This study opens the door to several intriguing questions,” according to a Commentary in the journal by sleep specialists Jonathan Jun, MD, and Vsevolod Polotsky, MD, PhD, of Johns Hopkins University School of Medicine. Could variations in individual responses to short sleep explain susceptibility to metabolic consequences? Could dysregulation of fatty acid metabolism represent a common pathway linking various sleep disorders to metabolic syndrome? And why don’t clinicians routinely ask their patients about sleep?
The study provides evidence for “potential mechanisms by which sleep restriction may be associated with insulin resistance and increased type 2 diabetes risk,” the authors conclude. It supports the growing sense that insufficient sleep may disrupt fat metabolism. And it suggests that an intervention as simple as getting enough sleep could counteract the current epidemics of diabetes and obesity.
Story Source:
The above story is based on materials provided by University of Chicago Medical Center. Note: Materials may be edited for content and length.
Journal Reference:
- Esra Tasali et al. Sleep restriction increases free fatty acids in healthy men. Diabetologia, February 2015 DOI: 10.1007/s00125-015-3500-4