On Dec. 26, 2013, a two-year-old boy living in the Guinean village of Meliandou, Guéckédou Prefecture was stricken with a rare disease, caused by the filament-shaped Ebola virus.
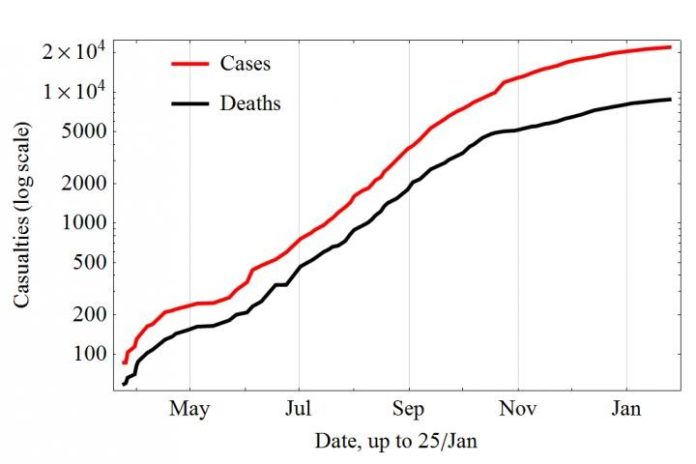
The child is believed to be the first case in what soon became a flood-tide of contagion, ravaging the West African countries of Guinea, Sierra Leone and Liberia, infecting, according to the World Health Organization, over 21,000 cases as of Jan. 21, with nearly 9000 confirmed deaths–the actual toll likely much higher.
Now, researchers from Arizona State University and Georgia State University are trying to better understand the epidemiology and control of Ebola Virus Disease in order to alleviate suffering and prevent future disease outbreaks from reaching the catastrophic proportions of the current crisis.
In reports appearing in the February 2015 issue of the British medical journal The Lancet Infectious Disease, ASU researchers report on new efforts to model the impact of timely diagnostic testing on the spread of Ebola across populations. A better understanding of viral dissemination and techniques for disease management are vital if a similar calamity is to be avoided in the future.
Researchers from the Biodesign Institute, and the Simon A. Levin Mathematical, Computational and Modeling Sciences Center present a new study: Modelling the effect of early detection of Ebola. The study examines the levels of detection and patient isolation required to shut down transmission of Ebola.
In related research, Gerardo Chowell, a newly appointed faculty member in the School of Public Health at Georgia State University and adjunct faculty member in the ASU’s Simon A. Levin Mathematical, Computational and Modeling Sciences Center, together with Cécile Viboud from the from the National Institutes of Health discuss recent large-scale modeling efforts to explain the spatial-temporal patterns of spread of the epidemic in Liberia. Chowell is also co-author of Ebola control: rapid diagnostic testing, which appears in the Lancet‘s correspondence section.
Wave of destruction
The Ebola virus has become notorious, not only for its highly contagious and lethal nature, but for the nightmarish assortment of symptoms collectively known as hemorrhagic fever. These may include vomiting of blood, bleeding from the eyes, ears, nose, mouth, rectum, internal bleeding, excruciating pain and the liquidization of internal organs.
The three West African nations centrally affected by the epidemic were acutely unprepared for the crisis. Treatment centers were rapidly swamped with severely ill patients. Resources for proper care, isolation of infected patients and even basic means of sterilization were soon depleted. Health care workers were especially vulnerable to infection.
A number of exacerbating factors contributed to the outbreak and rapid spread of Ebola in the region. Timber and mining activities have impacted densely forested regions and brought fruit bats–believed to be a natural reservoir for the virus–in closer contact with humans. Infected animals consumed as bush meat may also have planted early seeds of the disease in the vulnerable population. Long periods of civil unrest have left the area deeply impoverished and the health infrastructure fractured.
Meliandou, the town identified as ground zero, is situated in a forested area at the convergence point of Guinea, Liberia and Sierra Leone. Populations move fluidly across these porous borders, as impoverished residents are often on the move in search of work. These conditions created a perfect storm for the aggressive virus.
An additional factor fueling the explosive spread of Ebola in West Africa was the delayed and inadequate response to the crisis on the part of developed countries and global health organizations.
Time is the enemy
As the authors of the Lancet modeling study emphasize, breaking the chain of Ebola transmission presents intimidating challenges. After the development of symptoms, the virus is highly contagious and each new contact presents an opportunity for further spread of the disease.
Tracking all contacts of infected individuals can be a daunting challenge, even in first world settings, with low case numbers. In the absence of a vaccine or reliable therapeutic for Ebola, diagnosis of the disease at a pre-symptomatic stage and rapid isolation of infected individuals are the surest means for arresting further disease transmission.
According to Biodesign’s Karen Anderson, PhD., “Early detection of Ebola infection provides the opportunity and time to safely isolate and treat individuals before they become contagious. Our findings show two key things: first, that the predicted impact of early diagnostic tests depends on existing public health measures. Second, there appears to be a tipping point, where early diagnosis of high-risk individuals, combined with adequate isolation, can markedly decrease the predicted number of infected individuals.”
Stopping an epidemic in its tracks requires a reduction in a critical value known as the reproductive ratio or R0– a measure of new infections generated by a single case over the course of the infectious period. The higher the number for R0, the more difficult an epidemic is to contain.
A technique known as polymerase chain reaction (PCR) can be used for pre-symptomatic identification of the Ebola virus. The current study models the expected outcomes on viral transmission of Ebola using PCR-based pre-symptomatic diagnosis and isolation of infected patients within 3 days of the onset of symptoms.
“Our results underscore the dramatic impact that diagnostic capacity can bring about during an Ebola epidemic to quickly identify Ebola cases before these start new chains of transmission in the community or health care settings,” according to Diego Chowell, lead author of the study.
Carlos Castillo-Chavez, director of the Simon A. Levin Mathematical, Computational and Modeling Sciences Center, emphasises the power of mathematical modeling for understanding and limiting the scale of epidemics: “Finding that small differences in isolation effectiveness may have a large impact on epidemic size highlights the importance of evaluating novel diagnostic technologies at the population level using mathematical models,” he says. “An intervention may not work or be effective unless it is effectively used beyond a tipping point.”
The authors urge the implementation of the strategy of pre-symptomatic diagnosis and rapid isolation, targeting high-risk individuals, including care givers and health care workers.
In his comment to the Lancet, Geraldo Chowell examines another mathematical model, put forward by Merler and his colleagues. This study models the course of the Ebola epidemic in Liberia, based on population structure and geography, including location of households, hospitals, and Ebola treatment units.
Chowell notes that the establishment of new treatment centers, isolation of new patients and distribution of household protection kits all likely played a role in curtailing the spread of Ebola in Liberia, relative to neighboring states of Guinea and Sierra Leone.
“Carefully calibrated mathematical models have potential to guide public health authorities to effectively respond to disease epidemics,” Chowell says. “In the context of the Ebola epidemic in West Africa, several key factors, including delays in responding to the epidemic, behavior changes and increased public health infrastructure in the region in order to trace contacts of infected individuals and break chains of transmission through effective isolation have played a major role in shaping the trajectory of this epidemic.”
The desperate need for early diagnosis of Ebola was further emphasized in Chowell’s correspondence, which points out that most West African Ebola patients remained undiagnosed in their communities and the average time from symptom onset to diagnosis was about 5 days–a prescription for rapid, far-flung transmission of the disease.
While underscoring the diagnostic power of PCR, Chowell notes that such tests presently require transportation to a laboratory or transit center, causing critical delays in diagnosis and treatment and heightening transmission risks. His recommendation is to supplement these efforts with the distribution of point-of-care rapid tests that could be used in households for early protection.
Chowell and his colleagues conducted a simulation based on reducing the time between symptom onset and diagnosis, using rapid testing. The results were dramatic. If 60 percent of Ebola patients can be rapidly diagnosed and isolated (within 1 day of symptom onset), the proportion of the population eventually infected (known as the attack rate) drops from 80 percent to nearly zero.
A vaccine for Ebola?
While authors of the current Lancet papers model Ebola transmission and propose strategies to address future epidemics, ASU has also been on the forefront of efforts toward Ebola therapeutics and eventual vaccines.
Charles Arntzen, Ph.D., offered a prescient warning back in 2011 that the next outbreak of Ebola could be far more devastating than those in the past, if sufficient resources were not immediately brought to bear.
Regrettably, Arntzen’s prediction became a reality with the recent epidemic, far surpassing in death toll and geographic extent all previous Ebola outbreaks combined.
Arntzen’s earlier study in the Proceedings of the National Academy of Science described an experimental cocktail of monoclonal antibodies produced from tobacco plants, which showed considerable promise in animal studies.
The recent West African epidemic provided an unprecedented opportunity to test the effectiveness of the drug formula, developed with Arntzen’s longtime collaborators at San Diego based MAPP Pharmaceuticals. Two health care workers returning to the U.S. after having been stricken with Ebola in Africa were treated with the drug, known as ZMapp. Both survived, offering the tantalizing potential for a safe, highly effective vaccine against the disease.
Arntzen’s efforts also highlighted the potential of similar plant-made pharmaceuticals. A number of these are currently being investigated at the Biodesign Institute by Qiang “Shawn” Chen, Ph.D., a researcher in the Center for Infectious Diseases and Vaccinology. Chen hopes to apply similar techniques to produce therapeutics against other diseases, including West Nile Fever, a focus of current research.
According to the latest reports from the World Health Organization, the Ebola epidemic appears to be weakening its grip on the region. For the first time since June 2014, there have been fewer than 100 new weekly cases reported in the 3 countries most affected, signaling what health care workers hope is the final phase of Ebola’s devastating reign.
Increased vigilance and new tools at both the epidemiological and therapeutic ends of the spectrum are vitally needed, if another epidemic–perhaps of even greater scale–is to be prevented.
Story Source:
The above story is based on materials provided by Arizona State University. Note: Materials may be edited for content and length.
Journal Reference:
- Diego Chowell, Carlos Castillo-Chavez, Sri Krishna, Xiangguo Qiu, Karen S Anderson. Modelling the effect of early detection of Ebola. The Lancet Infectious Diseases, 2015; 15 (2): 148 DOI: 10.1016/S1473-3099(14)71084-9