According to the Urology Care Foundation, the official foundation of the American Urological Association, 33 million Americans suffer from overactive bladder (OAB). That’s 30% of all men and 40% of all women in the United States. The foundation estimates that the actual number is much larger because many people who have overactive bladder problems are embarrassed and do not seek care.
This represents a nearly twofold increase since 2001 when a paper written said 17 million people had the problem.
Its’ not clear how either of the two sources cited above obtained their information.
Maybe you didn’t know that there are two kinds of OAB. “Dry” is the one where the patient is able to get to the bathroom on time. “Wet” is the form that is accompanied by leakage of urine also known as the urge incontinence.
Here is something else you probably didn’t know. The disease was virtually unknown before 1997.
What happened in that year?
A drug company, Pharmacia, had a drug called Detrol that they were originally going to market for the treatment of urinary incontinence. However the number of people with urinary incontinence was small. They decided to focus on people who had urinary frequency and urgency and declared that incontinence was not absolutely necessary to make the diagnosis of OAB.
As related in the book “Our Daily Meds,” the drug company had to convince people who went to the restroom frequently and were previously treated with conservative measures such as restricting fluid intake and eliminating caffeine from the diet that OAB was not just an annoyance or inconvenience but a serious condition requiring professional medical care. They named it “Overactive Bladder.”
A special supplement to the journal Urology in 1997 contained 30 articles about OAB, a number of which were written by doctors who were paid by Pharmacia.
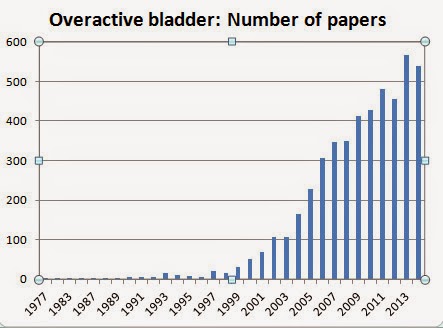
Here is a graph by year for the 4803 papers listed in PubMed on the subject of OAB since the term first appeared in 1977.
 Of the 69 papers written before 1997, only 7 used the term “overactive bladder.”
Of the 69 papers written before 1997, only 7 used the term “overactive bladder.”
There are now seven drugs for the treatment of OAB on the market. Consumer Reports says that none of the seven is clearly more effective than the others. The monthly cost of these drugs can be as low as $4.00 for a generic to $300 for a brand name medication.
In 2010, the website Decision Resources predicted that “the OAB drug market will increase from approximately $3 billion in 2009 to nearly $4 billion in 2019 in the United States, France, Germany, Italy, Spain, United Kingdom and Japan.”
Not a bad return for treating a disease that did not even exist until 18 years ago.