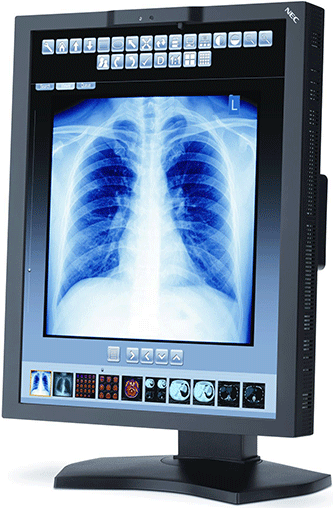
NEC announced receiving FDA clearance for its MultiSync MD210C3 monitor to be used for diagnostic applications. At $4,299, the 3-megapixel LED display is promoted as being “cost-effective” and has a 21.3″ screen with an integrated front sensor to keep it constantly calibrated.
NEC announced receiving FDA clearance for its MultiSync MD210C3 monitor to be used for diagnostic applications. At $4,299, the 3-megapixel LED display is promoted as being “cost-effective” and has a 21.3″ screen with an integrated front sensor to keep it constantly calibrated.
There’s also a human presence sensor that will dim the screen if there’s no one in front for both patient data privacy and energy savings. It has a built-in USB hub and accepts DisplayPort and Dual Link DVI connections.
Some features according to the product page:
- Out-of-the-box calibration to the DICOM grayscale display function for luminance
- Integrated tri-stimulus (three-color) sensor receives more light information than standard (brightness only) sensors and, therefore, is extremely accurate and stable
- GammaComp™ MD software ensures consistent image quality, while providing a simple interface for conformance to the DICOM standard and an easy-to-use QA environment for medical imaging
- 14-bit 3D internal programmable lookup tables (LUTs) enable precise calibration
- Auto luminance control with backlight stabilization for consistent brightness and color
- Built-in ambient light sensor automatically adjusts the display’s brightness based on existing lighting conditions, reducing energy consumption
- Quick QA feature allows for a one-button check of DICOM conformance
Product page: MultiSync MD210C3…
Press release: NEC DISPLAY SOLUTIONS RECEIVES FDA 510(K) CLEARANCE ON MD210C3 DIAGNOSTIC REVIEW MONITOR…
The post NEC MultiSync MD210C3 Clinical Monitor Lands FDA Clearance appeared first on Medgadget.