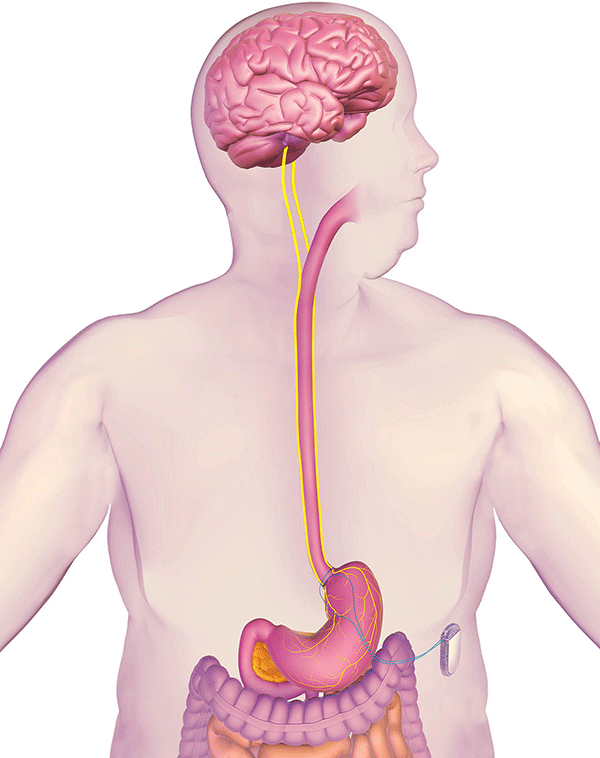
EnteroMedics, a company out of St. Paul, Minnesota, announced that its Maestro VBLOC vagal blocking therapy system can now be made available in the U.S. for people on the front lines of the war with obesity. The device intermittently blocks vagus nerve trunks by sending electronic pulses that interfere with natural signals coming from the stomach.
 The system is indicated for patients with a body mass index (BMI) of at least 40 to 45 kg/m2, or a BMI of at least 35 to 39.9 kg/m2 coupled with additional condition such as hypertension or high cholesterol. The patients must have also gone through a supervised weight management program within the past five years with little success.
The system is indicated for patients with a body mass index (BMI) of at least 40 to 45 kg/m2, or a BMI of at least 35 to 39.9 kg/m2 coupled with additional condition such as hypertension or high cholesterol. The patients must have also gone through a supervised weight management program within the past five years with little success.
The implantation can be done in an outpatient procedure and, since the native anatomy is not altered, it can be completely reversed and the device explanted as necessary.
Some details on what led to the approval:
Approval of the Maestro Rechargeable System was based on the ReCharge Study, a randomized, double-blind, sham-controlled trial to evaluate the safety and effectiveness of the Maestro Rechargeable System in treating obesity. In an intention to treat (ITT) analysis of the study results, VBLOC-treated patients achieved 24.4% excess weight loss (EWL) at 12 months. At 18 months, VBLOC-treated patients maintained a 23.5% EWL. In a responder analysis of the ITT population at 12 months, over 50% of VBLOC-treated patients achieved 20% or greater EWL.
The SAE (severe adverse event) rate, defined as the proportion of subjects in the VBLOC treated group who experienced an implant/revision procedure, device or therapy-related SAE through 12 months post-implant, was 3.7% (n=6; 95% CI: 1.4% to 7.9%) in the ITT population. The most common ( > 10%) non-serious adverse events related to device, implant/revision procedure or therapy were pain at the neuroregulator site, and transient sensations of therapy such as heartburn/dyspepsia.
VBLOC Therapy is contraindicated for use in patients with cirrhosis of the liver, portal hypertension, esophageal varices or an uncorrectable, clinically significant hiatal hernia; patients for whom magnetic resonance imaging (MRI) or diathermy use is planned; patients at high risk for surgical complications; and patients who have a permanently implanted, electrical-powered medical device or gastrointestinal device or prosthesis (e.g. pacemakers, implanted defibrillators, neurostimulators).
Flashbacks: EnteroMedics Presents Updated VBLOC Trial Data…; Vagal Electrical Nerve Stimulation Helps With Weight Loss in Clinical Trial…; CE Mark Awarded to EnteroMedics’ Next Generation Obesity Therapy Stimulator…; European Union Clears VBLOC Vagal Nerve Blocking Therapy for Obesity Control…
Product page: Maestro System…
Press release: EnteroMedics Announces FDA Approval of VBLOC® Vagal Blocking Therapy for the Treatment of Obesity…
The post Enteromedics Clinches FDA Approval for Nerve Stimulator to Treat Obesity appeared first on Medgadget.