BD Intelliport Syringe Labeler
BD Medical, a part of Becton, Dickinson and Company, announced receiving FDA clearance for its Intelliport Medication Management System. The product monitors manual IV bolus injections, automatically records how much of which medication a patient receives and warns of allergies by interfacing with the hospital’s electronic medical records.
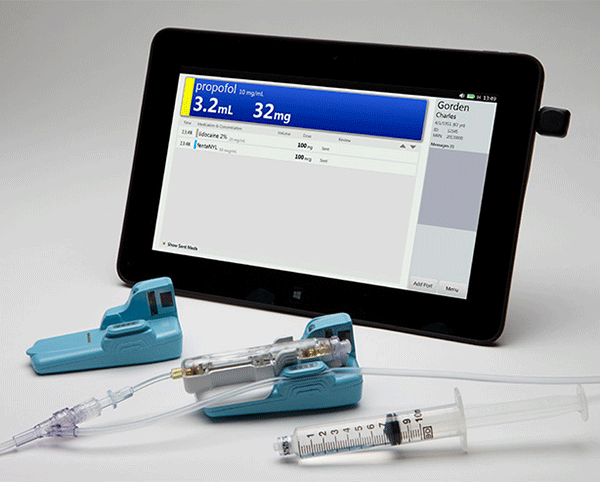
The system comes with an IV site sensor, a wireless base, and a Windows-based touch-screen tablet. The base and sensor scan the barcode on the vial and measure the amount of medication that is injected. The base is non-sterile and reusable, while the sensor that goes inside of it is single-patient use. There’s also a matching syringe labeler that sticks barcoded stickers to the luer tips of syringes to make everything work together.
BD is expecting that the product will be available in the spring of this year.
Here’s a BD video presenting the Intelliport:
Product page: Intelliport Medication Management System…
Press release: The BD™ Intelliport® Medication Management System Receives Clearance from the Food and Drug Administration (FDA)…
The post BD Intelliport IV Medication Management System Cleared by FDA (VIDEO) appeared first on Medgadget.