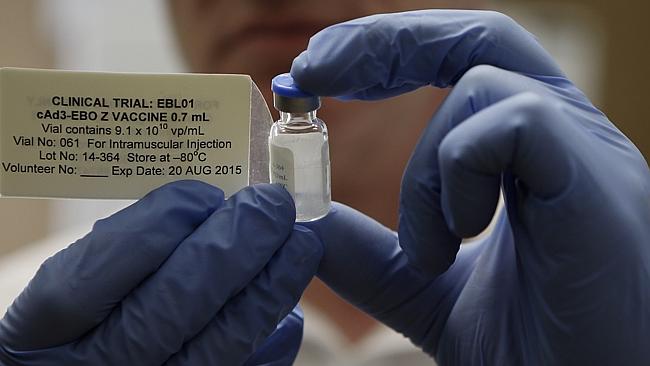
Two vaccines for Ebola are ready to be deployed in frontline trials in West Africa, the World Health Organization said Friday.
After convening an expert meeting this week, WHO said the two leading vaccine candidates, produced by GlaxoSmithKline and Merck & Co, had both shown an “acceptable safety profile” as well as the potential to stimulate the immune system against Ebola in initial trials in Europe and North America, clearing the way for trials evaluating their effectiveness.
Late-stage trials are expected to begin in Liberia by the end of this month, and in Sierra Leone and Guinea in February, involving more than 40,000 people — including healthcare workers and healthy volunteers. Only after these have been completed in about six months will it be clear whether the products work.
Praising the companies and scientists who had rushed the products to the brink of large-scale trials, WHO Director-General Dr. Margaret Chan said they had given themselves tight deadlines and were moving ahead in record time. “In fact, what [they] are doing is unprecedented: compressing into a matter of months work that normally takes two to four years, yet with no compromise of international standards of safety and efficacy,” she said.
The first trial in Liberia will involve three groups of 9,000 people. One will get the GSK vaccine, another will get the Merck shot and a third will get a placebo. Further trials involving about 6,000 people in Sierra Leone and 9,000 in Guinea will start next month.
A third vaccine, developed by Johnson & Johnson, entered initial clinical trials in the UK this week with large-scale studies expected by April. The US company, which is collaborating with Denmark’s Bavarian Nordic, said it had already produced enough vaccine to treat more than 400,000 people with the potential to produce a total of 2m courses by the end of this year.
Trials of the Merck vaccine were briefly halted last month after participants reported joint pain, but the WHO said this side effect had since been deemed tolerable. The UN agency also noted that more work is needed to decide what doses of the two lead vaccines should be given, but said this should be completed within the next few weeks.
Meanwhile, researchers at Oxford University in Britain announced this week that they were starting safety trials of another vaccine candidate. Unlike the other experimental vaccines, this one uses two separate injections — a “prime-boost” shot to stimulate the immune system and a booster shot a few weeks later to enhance immune response over time. The Oxford team said they plan to vaccinate 72 healthy adults with the experimental vaccine, developed by Johnson & Johnson, by the end of January.
More than 8,000 people have died out of almost 21,000 cases of Ebola since last March — by far the worst outbreak of the virus since it was discovered 39 years ago. According to the WHO’s latest situation assessment, the rate of new infections has fallen in Liberia and appears to be stabilizing in Sierra Leone and Guinea.