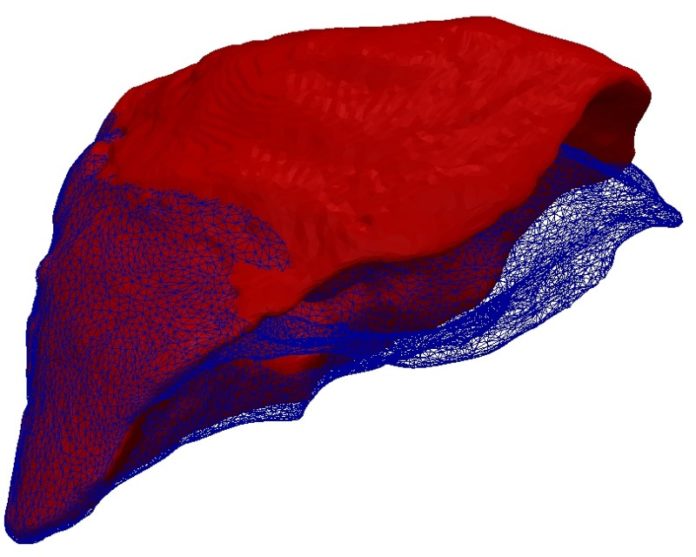
During minimally invasive operations, a surgeon has to trust the information displayed on the screen: A virtual 3D model of the respective organ shows where a tumor is located and where sensitive vessels can be found. Soft tissue, such as the tissue of the liver, however, deforms during breathing or when the scalpel is applied. Endoscopic cameras record in real time how the surface deforms, but do not show the deformation of deeper structures such as tumors. Young scientists of the Karlsruhe Institute of Technology (KIT) have now developed a real-time capable computation method to adapt the virtual organ to the deformed surface profile.
The principle appears to be simple: Based on computer tomography image data, the scientists construct a virtual 3D model of the respective organ, including the tumor, prior to operation. During the operation, cameras scan the surface of the organ and generate a stiff profile mask. To this virtual mold, the 3D model then is to fit snuggly, like jelly to a given form. The Young Investigator Group of Dr. Stefanie Speidel analyzed this geometrical problem of shape adaptation from the physical perspective. “We model the surface profile as electrically negative and the volume model of the organ as electrically positive charged,” Speidel explains. “Now, both attract each other and the elastic volume model slides into the immovable profile mask.” The adapted 3D model then reveals to the surgeon how the tumor has moved with the deformation of the organ.
Simulations and experiments using a close-to-reality phantom liver have demonstrated that the electrostatic-elastic method even works when only parts of the deformed surface profile are available. This is the usual situation at the hospital. The human liver is surrounded by other organs and, hence, only partly visible by endoscopic cameras. “Only those structures that are clearly identified as parts of the liver by our system are assigned an electric charge,” says Dr. Stefan Suwelack who, as part of Speidel’s group, wrote his Ph.D. thesis on this subject. Problems only arise, if far less than half of the deformed surface is visible. To stabilize computation in such cases, the KIT researchers can use clear reference points, such as crossing vessels. Their method, however, in contrary to others does not rely on such references from the outset.
In addition, the model of the KIT researchers is more precise than conventional methods, because it also considers biomechanical factors of the liver, such as the elasticity of the tissue. So for instance, the phantom liver used by the scientists consists of two different silicones: A harder material for the capsule, i.e. the outer shell of the liver, and a softer material for the inner liver tissue.
As a result of their physical approach, the young scientists also succeeded in accelerating the computation process. As shape adaptation was described by electrostatic and elastic energies, they found a single mathematical formula. Using this formula, even conventional computers equipped with a single processing unit only work so quickly that the method is competitive. Contrary to conventional computation methods, however, the new method is also suited for parallel computers. Using such a computer, the Young Investigator Group now plans to model organ deformations stably in real time.
Story Source:
The above story is based on materials provided by Karlsruhe Institute of Technology. Note: Materials may be edited for content and length.
Journal Reference:
- Stefan Suwelack, Sebastian Röhl, Sebastian Bodenstedt, Daniel Reichard, Rüdiger Dillmann, Thiago dos Santos, Lena Maier-Hein, Martin Wagner, Josephine Wünscher, Hannes Kenngott, Beat P. Müller, Stefanie Speidel. Physics-based shape matching for intraoperative image guidance. Medical Physics, 2014; 41 (11): 111901 DOI: 10.1118/1.4896021