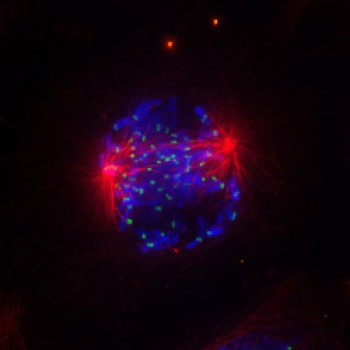
A Danish Research Team identified a molecular mechanism that ensures that when cells divide, the genomic material passes correctly to the resulting daughter cells: “The process, known as chromosome segregation, is vitally important because incorrect passage of the genomic material makes cells prone to develop into cancer cells,” says Jakob Nilsson, associate professor at the Novo Nordisk Foundation Center for Protein Research. The new discovery depends on a protein called BubR1 which if mutated can cause cancer.
The results have just been published in the scientific journal Nature Communications.
Chromosome segregation
Recently there has been a lot of interest in understanding the chromosome segregation process and the impact of the protein BubR1. The research team used sophisticated live cell microscopy to follow chromosome movements in human cells that had mutations in the BubR1 protein, in order to see how this affected chromosome segregation. This allowed the scientists to uncover novel aspects of how BubR1 ensures correct chromosomes segregation and how BubR1 mutations affect the response to chemotherapeutic agents used in the clinic.
“We know that BubR1 is mutated in certain human cancers so our work could help explain how BubR1 causes cancer when mutated. In the future it could be beneficial to analyze cancer patients for mutations in BubR1 and the insight we have gained with this study will allow us to predict the outcome of such mutations,” says Jakob Nilsson, Associate Professor from the Novo Nordisk Foundation Center for Protein Research.
Potential benefits
The hope is that this research will help in defining better strategies for cancer therapy and provide better understanding of why chromosomes are often not correctly passed on to the new daughter cells in cancer.
“Today, cancer treatment is often based on trial and error but in the future we hope to predict patients’ responses to various drug treatments, based on knowing the mutations present in the individual cancer tissue. Simply put, patients with mutations in BubR1 could be responsive to specific types of drugs and ideally, by analyzing patients for the presence of mutated BubR1 and other biomarkers, we will know which drug to use in advance,” adds Jakob Nilsson.
Story Source:
The above story is based on materials provided by University of Copenhagen – The Faculty of Health and Medical Sciences. Note: Materials may be edited for content and length.
Journal Reference:
- Tiziana Lischetti, Gang Zhang, Garry G. Sedgwick, Victor M. Bolanos-Garcia, Jakob Nilsson. The internal Cdc20 binding site in BubR1 facilitates both spindle assembly checkpoint signalling and silencing. Nature Communications, 2014; 5: 5563 DOI: 10.1038/ncomms6563