An international research collaborative has determined that a promising anti-malarial compound tricks the immune system to rapidly destroy red blood cells infected with the malaria parasite but leave healthy cells unharmed. St. Jude Children’s Research Hospital scientists led the study, which appears in the current online early edition of the Proceedings of the National Academy of Sciences (PNAS).
The compound, (+)-SJ733, was developed from a molecule identified in a previous St. Jude-led study that helped to jumpstart worldwide anti-malarial drug development efforts. Malaria is caused by a parasite spread through the bite of an infected mosquito. The disease remains a major health threat to more than half the world’s population, particularly children. The World Health Organization estimates that in Africa a child dies of malaria every minute.
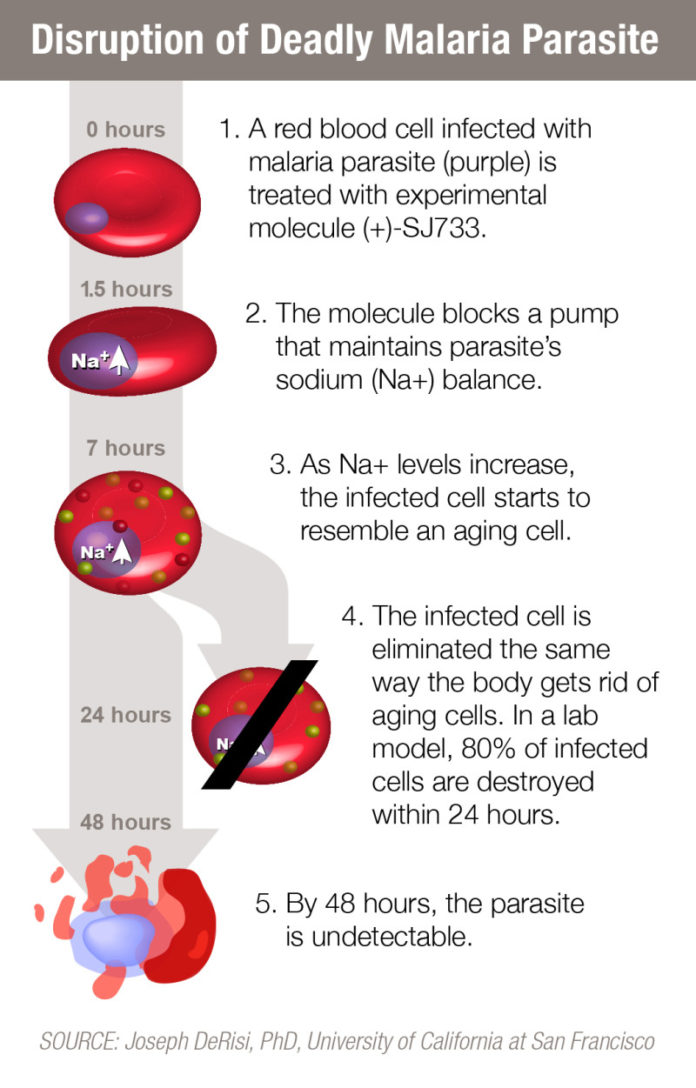
In this study, researchers determined that (+)-SJ733 uses a novel mechanism to kill the parasite by recruiting the immune system to eliminate malaria-infected red blood cells. In a mouse model of malaria, a single dose of (+)-SJ733 killed 80 percent of malaria parasites within 24 hours. After 48 hours the parasite was undetectable.
Planning has begun for safety trials of the compound in healthy adults.
Laboratory evidence suggests that the compound’s speed and mode of action work together to slow and suppress development of drug-resistant parasites. Drug resistance has long undermined efforts to treat and block malaria transmission.
“Our goal is to develop an affordable, fast-acting combination therapy that cures malaria with a single dose,” said corresponding author R. Kiplin Guy, Ph.D., chair of the St. Jude Department of Chemical Biology and Therapeutics. “These results indicate that SJ733 and other compounds that act in a similar fashion are highly attractive additions to the global malaria eradication campaign, which would mean so much for the world’s children, who are central to the mission of St. Jude.”
Whole genome sequencing of the Plasmodium falciparum, the deadliest of the malaria parasites, revealed that (+)-SJ733 disrupted activity of the ATP4 protein in the parasites. The protein functions as a pump that the parasites depend on to maintain the proper sodium balance by removing excess sodium.
The sequencing effort was led by co-author Joseph DeRisi, Ph.D., a Howard Hughes Medical Institute investigator and chair of the University of California, San Francisco Department of Biochemistry and Biophysics. Investigators used the laboratory technique to determine the makeup of the DNA molecule in different strains of the malaria parasite.
Researchers showed that inhibiting ATP4 triggered a series of changes in malaria-infected red blood cells that marked them for destruction by the immune system. The infected cells changed shape and shrank in size. They also became more rigid and exhibited other alterations typical of aging red blood cells. The immune system responded using the same mechanism the body relies on to rid itself of aging red blood cells.
Another promising class of antimalarial compounds triggered the same changes in red blood cells infected with the malaria parasite, researchers reported. The drugs, called spiroindolones, also target the ATP4 protein. The drugs include NITD246, which is already in clinical trials for treatment of malaria. Those trials involve investigators at other institutions.
“The data suggest that compounds targeting ATP4 induce physical changes in the infected red blood cells that allow the immune system or erythrocyte quality control mechanisms to recognize and rapidly eliminate infected cells,” DeRisi said. “This rapid clearance response depends on the presence of both the parasite and the investigational drug. That is important because it leaves uninfected red blood cells, also known as erythrocytes, unharmed.”
Laboratory evidence also suggests that the mechanism will slow and suppress development of drug-resistant strains of the parasite, researchers said.
Planning has begun to move (+)-SJ733 from the laboratory into the clinic beginning with a safety study of the drug in healthy adults. The drug development effort is being led by a consortium that includes scientists at St. Jude, the Swiss-based non-profit Medicines for Malaria Venture and Eisai Co., a Japanese pharmaceutical company.
Story Source:
The above story is based on materials provided by St. Jude Children’s Research Hospital. Note: Materials may be edited for content and length.
Journal Reference:
- María Belén Jiménez-Díaz, Daniel Ebert, Yandira Salinas, Anupam Pradhan, Adele M. Lehane, Marie-Eve Myrand-Lapierre, Kathleen G. O’Loughlin, David M. Shackleford, Mariana Justino de Almeida, Angela K. Carrillo, Julie A. Clark, Adelaide S. M. Dennis, Jonathon Diep, Xiaoyan Deng, Sandra Duffy, Aaron N. Endsley, Greg Fedewa, W. Armand Guiguemde, María G. Gómez, Gloria Holbrook, Jeremy Horst, Charles C. Kim, Jian Liu, Marcus C. S. Lee, Amy Matheny, María Santos Martínez, Gregory Miller, Ane Rodríguez-Alejandre, Laura Sanz, Martina Sigal, Natalie J. Spillman, Philip D. Stein, Zheng Wang, Fangyi Zhu, David Waterson, Spencer Knapp, Anang Shelat, Vicky M. Avery, David A. Fidock, Francisco-Javier Gamo, Susan A. Charman, Jon C. Mirsalis, Hongshen Ma, Santiago Ferrer, Kiaran Kirk, Iñigo Angulo-Barturen, Dennis E. Kyle, Joseph L. DeRisi, David M. Floyd, R. Kiplin Guy. ( )-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance ofPlasmodium. Proceedings of the National Academy of Sciences, 2014; 201414221 DOI: 10.1073/pnas.1414221111