Researchers at the Mechanobiology Institute have comprehensively described the network of proteins involved in cell-cell adhesions, or the cadherin interactome. This work was published in Science Signaling (Guo et al. E-cadherin interactome complexity and robustness resolved by quantitative proteomics, Science Signaling, 02 Dec 2014, Vol 7, Issue 354).
Unlocking the complexity of cell adhesion
Many biological processes depend on the ability of cells to stick to one another. The formation of multicellular organisms and precise embryonic development rely on this property, as does the maintenance of healthy tissue. Defects in the ability of cells to adhere to one another have been found in many diseases, such as cancer, Alzheimer’s disease and cardiovascular disease. In the case of cancer, ineffective cell adhesion allows tumour cells to detach and invade other tissues, thereby spreading cancer throughout the body.
Cell-cell adhesion is made possible through various cellular structures that are collectively known as cell-cell adhesion complexes. The most prominent cell-cell adhesion complex is the Adherens Junction. Central to adherens junctions is a protein known as E-cadherin, or epithelial cadherin. E-cadherin spans the cell membrane, providing a link between the interior, and exterior of the cell. Outside the cell, E-cadherin binds to other E-cadherins from neighbouring cells in a mechanism that can be described as a ‘cellular handshake’. On the inside of the cell, E-cadherin binds to linker proteins known as catenins, which attach to a structural scaffold that lies adjacent to the adhesion site, the actin cytoskeleton. This physical link between the cytoskeletons of neighboring cells allows for the generation and transduction of mechanical signals.
Despite their importance in cell-cell adhesion, scientists have yet to fully understand how the cadherin-catenin-actin complex forms and is regulated. To extend the idea of cell adhesion being like a ‘cellular handshake’, imagine walking along a crowded street while holding hands with a partner. Moving together with the flow of people, navigating obstacles, adjusting your speed and responding to changes in conditions must all be considered if you are to reach your destination without letting go. Similarly, cells must maintain their adhesion while facing varying stresses and biochemical conditions. Hence, the adhesive structures are regulated and adjusted, via a complex network of structural and regulatory proteins. Where defective adhesion has led to a certain disease it is essential to understand where the problem lies and this requires stepping back and looking at the whole picture.
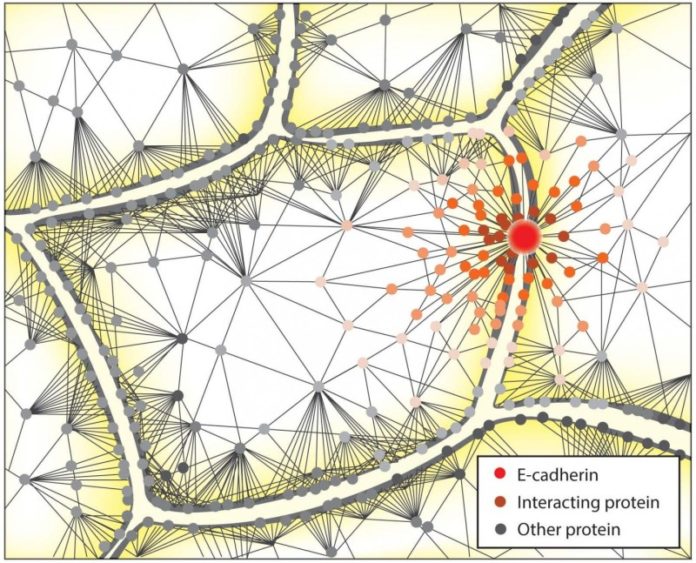
To better identify the components of this wider network in maintaining and regulating adhesion, researchers at the Mechanobiology Institute, National University of Singapore, applied a combination of experimental and computational techniques to reveal and dissect the complex network of proteins that interact with E-cadherin. To achieve this, E-cadherin was labelled with an enzyme that, when activated, releases a small cloud of a tagging molecule to flag all other proteins in the immediate vicinity. When coupled with quantitative proteomics, this provides a list of proteins interacting with E-cadherin, thus capturing many of the proteins that influence the adhesive properties of the cell.
Overall 561 proteins were found to be associated with E-cadherin, and remarkably 419 of these interactions were completely novel. Using a protein interaction database, the researchers created a map of the E-cadherin interactome that contains information on the function of each protein and its interactions with other proteins within the network. The majority of proteins found were identified as adaptor proteins, which serve as scaffolds within the Adherens Junction. Other proteins involved in cellular transport and protein synthesis were also identified. Interestingly, the researchers found that most of the proteins that associated with E-cadherin did so independently of cell-cell adhesion.
This study highlights that cell adhesion results not only from the formation of a cadherin-catenin-actin complex, but from the activity of more than 500 interacting proteins. Successful cell adhesion requires a cascade of events involving these proteins and any breakdown in this cascade could lead to impaired cell adhesion, and disease. With the E-cadherin interactome now described in detail, researchers can finally step back and view the complex picture that is cell-cell adhesion. This will allow disease related defects to be identified, and new targets researched to understand this vital biological process.
Story Source:
The above story is based on materials provided by National University of Singapore. Note: Materials may be edited for content and length.
Journal Reference:
- Z. Guo, L. J. Neilson, H. Zhong, P. S. Murray, S. Zanivan, R. Zaidel-Bar. E-cadherin interactome complexity and robustness resolved by quantitative proteomics. Science Signaling, 2014; 7 (354): rs7 DOI: 10.1126/scisignal.2005473