The analysis of drugs, natural products, and chemical substances found in the environment allows the identification of the chemical fragments responsible for a therapeutic or deleterious effect on human health.
This knowledge may be valuable for the design of drugs with fewer secondary effects, for associating diseases, and for identifying new uses for drugs currently on the market.
The predictive model developed by researchers at IRB Barcelona provides information for the treatment of 20% of human diseases.
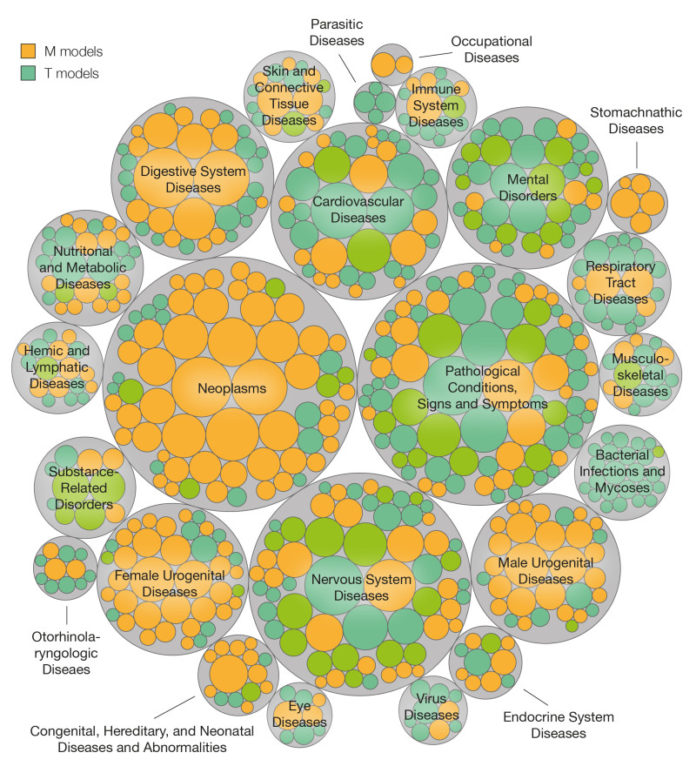
Current data bases hold information on thousands of molecules — including drugs, natural substances, and chemical agents found in the environment — that are associated with diseases, either because they have adverse effects or exert a therapeutic action. Using this information, gathered over many years and available in data bases, scientists headed by ICREA researcher Patrick Aloy at the Institute for Research in Biomedicine (IRB Barcelona) have devised a predictive model that allows them to associate chemical fragments with positive or negative effects in 20% of human diseases. Published in Nature Communications, the study may have applications for the design of safer drugs, the detection of comorbidity, and the extension of current drug uses.
The study has involved the analysis of 10,000 chemical molecules, which together comprise 98,077 fragments associated with 1,176 diseases — these representative of all human diseases.
Pharmacology prior to the ’80s seen from the 21st century
Until the ’80s, pharmacologists designed a chemical substance and studied its effect on a model organism. Without examining the impact of these substances at the molecular level or the proteins that they altered, these scientists developed many drugs for a wide range of diseases. This system of trial and error, which produced a large body of information, was abandoned with the advent of molecular biology. Since the beginning of the 21st century, drugs have been designed to alter the behaviour of proteins or genes that have previously been identified as being affected in certain diseases. Based on prior knowledge of biology, this approach is not producing the results expected.
The chemist Miquel Duran, a PhD student working on network biology at IRB Barcelona, questioned whether the accumulation of current data that associate the chemical structure of substances with disease through its therapeutic or deleterious effect would allow the development of a predictive model that related the chemical structure of a compound with its effect in humans. “In effect, we have enough information on chemical structures to reasonably predict their effects in 20% of human diseases,” explains Duran. “This implies the data on chemical structures, which on many occasions and above all in the field of molecular biology is not taken into account, can be most useful for biomedicine, for example for the design of safer drugs,” says the researcher. The study allows the information accumulated to be re-examined and reorganised and to produce 20% of new knowledge thanks to the exploitation of computerized data.
The design of safer drugs, prediction of disease co-morbidities and repositioning of drugs
Patrick Aloy explains that the study allows access to valuable information that can serve to avoid or foster the use of certain chemical fragments for drug design. For example, his model predicts that 40% of chemical fragments with therapeutic effects are not included in drugs currently on the market; in contrast, fragments that cause secondary effects are.
The researchers also indicate that their study may serve to detect associations between two diseases, the so-called co-morbidity — meaning one leads to the development of the other — or inverse comorbidity — namely that one protects against the development of the other. The study may also reveal potential new uses of current drugs.
Story Source:
The above story is based on materials provided by Institute for Research in Biomedicine-IRB. Note: Materials may be edited for content and length.
Journal Reference:
- Miquel Duran-Frigola, David Rossell, Patrick Aloy. A chemo-centric view of human health and disease. Nature Communications, 2014; 5: 5676 DOI: 10.1038/ncomms6676