Human existence is basically circadian. Most of us wake in the morning, sleep in the evening, and eat in between. Body temperature, metabolism, and hormone levels all fluctuate throughout the day, and it is increasingly clear that disruption of those cycles can lead to metabolic disease.
Underlying these circadian rhythms is a molecular clock built of DNA-binding proteins called transcription factors. These proteins control the oscillation of circadian genes, serving as the wheels and springs of the clock itself. Yet not all circadian cycles peak at the same time — some peak in the morning and others in the evening. The question, is, how does a single clock keep time in multiple phases at once? Now, thanks to new findings from researchers at the Perelman School of Medicine at the University of Pennsylvania, we know.
In the current issue of the journal Cell, Mitchell Lazar, MD PhD, the Sylvan Eisman Professor of Medicine and director of the Institute for Diabetes, Obesity, and Metabolism and his team report the results of a genome-wide survey of circadian genes and genetic regulatory elements called enhancers. These are key parts of the “dark matter” of the genome; rather than encoding proteins, they control the expression of genes.
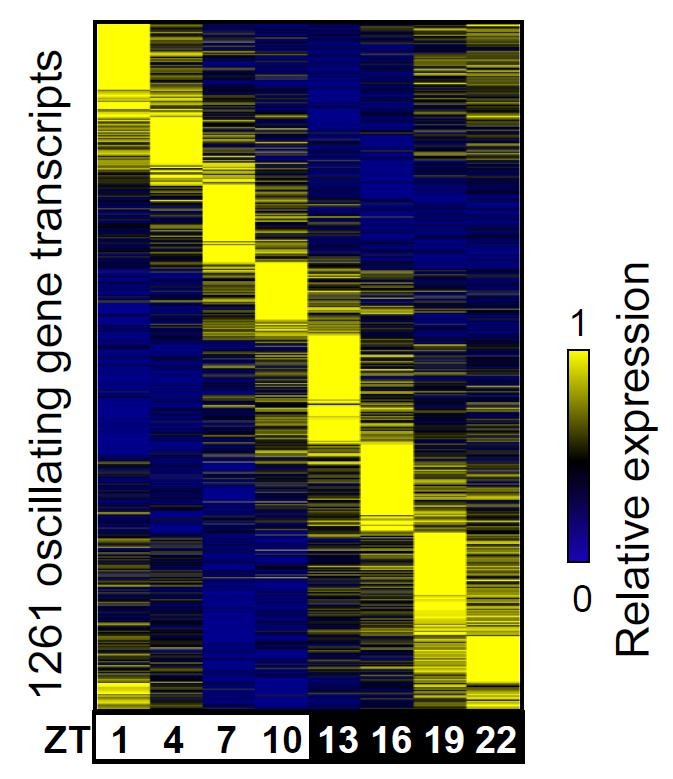
Led by postdoctoral researchers Bin Fang, Logan Everett and Jennifer Jager, Lazar’s team took advantage of new tools based on high-density DNA sequencing to measure the activity of enhancers throughout the day in the livers of mice. They found that many enhancers, like circadian genes themselves, have a daily oscillation that is in phase with nearby genes — both the enhancer and gene activity peak at the same time each day. The enhancer activities, in turn, are governed by distinct proteins called transcription factors. Grouping the enhancers into eight three-hour phases based on when they peak, the group asked which factors are capable of binding to the enhancers in each set. Remarkably, the team found that enhancers that are in the same phase tend to bind the same transcription factors.
For instance, a well-known circadian component called CLOCK binds enhancers that are most active during one particular three-hour period. Another protein called Rev-erb binds 12 hours later. Loss of any one of those factors dysregulates a subset of oscillating genes while leaving genes tuned to other circadian phases unaffected. “Different transcription factors control different phases,” Lazar concludes. “That explains how one clock leads to at least eight different subclocks.”
Importantly, genes that oscillate with the same phase tend to have related functions, which are distinct from the metabolic pathways controlled at other times. Genes involved in insulin signaling peak at a different time than genes that control sugar metabolism, for instance. Lazar suggests it might be possible to tweak pharmaceutical regimens to make them more efficient, by delivering drugs only when the pathways they impact are actually active — a strategy that could minimize unintended side effects, he says. “As a proof of concept for a new principle of drug treatment for metabolic disorders,” he says, “this is a real step in that direction.”
Story Source:
The above story is based on materials provided by Perelman School of Medicine at the University of Pennsylvania. Note: Materials may be edited for content and length.
Journal Reference:
- Bin Fang, Logan J. Everett, Jennifer Jager, Erika Briggs, Sean M. Armour, Dan Feng, Ankur Roy, Zachary Gerhart-Hines, Zheng Sun, Mitchell A. Lazar. Circadian Enhancers Coordinate Multiple Phases of Rhythmic Gene Transcription In Vivo. Cell, 2014; 159 (5): 1140 DOI: 10.1016/j.cell.2014.10.022