An international research consortium has discovered a new gene underlying progressive myoclonus epilepsy, one of the most devastating forms of epilepsy. The study showed that a single mutation in a potassium ion channel gene underlies a substantial proportion of unsolved cases. It is estimated that the mutation is carried by hundreds of patients worldwide. The study utilized modern DNA sequencing technologies, which have revolutionized genetic research of rare, severe diseases.
A study led by researchers at University of Helsinki, Finland and Universities of Melbourne and South Australia has identified a new gene for a progressive form of epilepsy. The findings of this international collaborative effort have been published today, 17 November 2014, in Nature Genetics.
Progressive myoclonus epilepsies (PME) are rare, inherited, and usually childhood-onset neurodegenerative diseases whose core symptoms are epileptic seizures and debilitating involuntary muscle twitching (myoclonus). The goal of the international collaborative study was to identify underlying genetic causes in 84 PME patients using DNA sequencing targeting the protein coding elements of the human genome.
Overall, a genetic diagnosis was reached for almost one third of these hitherto unsolved patients. Findings of the study shed light on molecular genetic basis of progressive epilepsy, which aids in diagnostics and potential therapeutic interventions of the disease. The study also shows that modern DNA technologies are powerful in dissecting the underlying genetic causes of severe diseases.
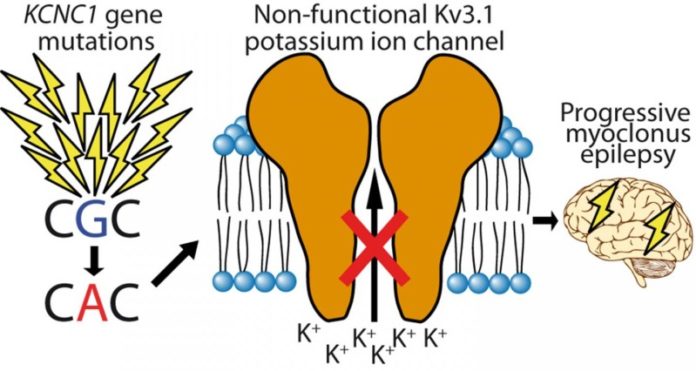
The most important and surprising finding of the study was that a single mutation in a potassium channel encoding gene KCNC1 explains a significant proportion of unsolved PME cases. The mutation was found in a total of 13 patients and was not inherited from parents — instead it has emerged in a germ cell of one of the parents or in the fertilized egg. Each individual has dozens of these new, “de novo”, mutations but they are rarely disease causing. The researchers estimate that this mutation occurs in approximately 1 out of every 5.7 million conceptions, indicating that at least hundreds of patients could have this mutation globally.
“A fascinating aspect of this finding is that this single mutation can be found in several patients all over the world. The mutation site is an example of a ‘mutation hotspot’ of the genome — a DNA nucleotide which is more prone for alterations,” says Professor Anna-Elina Lehesjoki, the corresponding principal investigator of the study in University of Helsinki and Folkhälsan Research Center, Finland.
The new mutation identified in the study disrupts the function of a potassium channel, KV3.1, which has a central role in signal transmission in the brain. The likely consequence of the mutation is that inhibitory signals in certain parts of patient brain are reduced, which makes patients susceptible to epileptic seizures and myoclonus starting in childhood. In addition, the mutation causes degeneration of the cerebellum and subtle cognitive decline in some of the patients.
“The fact that the mutation occurs in a well-characterized ion channel gives hope to development of targeted therapy. There are anti-epileptic drugs in the market that target other similar ion channels and follow-up research aims to discover a way to rescue the function of the channel in PME patients,” says Professor Lehesjoki.
The researchers of the project emphasize the importance of international collaboration for the study. “This study shows the power of combining sample collections and knowledge from different countries,” says Professor Samuel Berkovic, University of Melbourne, who coordinated the patient sample collection spanning over 20 years and involving multiple epilepsy centers worldwide.
The central research institutes participating in the study were University of Helsinki, Institute for Molecular Medicine Finland FIMM and Folkhälsan Research Center (Finland), Universities of Melbourne and South Australia (Australia), Wellcome Trust Sanger Institute (UK), University of Tübingen (Germany) and several universities in Italy.
Story Source:
The above story is based on materials provided by University of Helsinki. Note: Materials may be edited for content and length.
Journal Reference:
- Mikko Muona, Samuel F Berkovic, Leanne M Dibbens, Karen L Oliver, Snezana Maljevic, Marta A Bayly, Tarja Joensuu, Laura Canafoglia, Silvana Franceschetti, Roberto Michelucci, Salla Markkinen, Sarah E Heron, Michael S Hildebrand, Eva Andermann, Frederick Andermann, Antonio Gambardella, Paolo Tinuper, Laura Licchetta, Ingrid E Scheffer, Chiara Criscuolo, Alessandro Filla, Edoardo Ferlazzo, Jamil Ahmad, Adeel Ahmad, Betul Baykan, Edith Said, Meral Topcu, Patrizia Riguzzi, Mary D King, Cigdem Ozkara, Danielle M Andrade, Bernt A Engelsen, Arielle Crespel, Matthias Lindenau, Ebba Lohmann, Veronica Saletti, João Massano, Michael Privitera, Alberto J Espay, Birgit Kauffmann, Michael Duchowny, Rikke S Møller, Rachel Straussberg, Zaid Afawi, Bruria Ben-Zeev, Kaitlin E Samocha, Mark J Daly, Steven Petrou, Holger Lerche, Aarno Palotie, Anna-Elina Lehesjoki. A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy. Nature Genetics, 2014; DOI: 10.1038/ng.3144