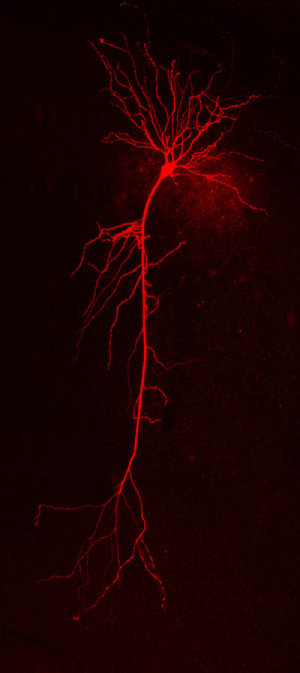
In diseases of the brain, such as Alzheimer’s and Parkinson’s, the neurons fail to communicate correctly with each other. As Bonn-based researchers of the German Center for Neurodegenerative Diseases (DZNE) now report in the journal “Neuron,” these connectivity problems can be ascribed to alterations in the structure of the nerve cells. For their study, the scientists investigated diseased nerve cells using high precision methods and subsequently simulated their electrical properties on the computer. In their view, medical interventions that preserve the structural integrity of neurons may constitute an innovative strategy for the treatment of neurodegenerative diseases.
Inside the brain, the nerve cells, which are also called “neurons,” are woven into a network in which they relay signals to one another. Thus, neurons form intricate projections that enable them to transmit electrical stimuli and synchronize their activity. “However, in Alzheimer’s, Parkinson’s and in other diseases of the brain, the nerve cells tend to atrophy. This is a typical symptom of neurodegenerative processes,” explains Professor Stefan Remy, who leads a research group at the Bonn site of the DZNE and also works for the Department of Epileptology at the University Hospital Bonn. “In general, diseased cells have smaller as well as fewer extensions than healthy cells.”
Troubles in Communication
It is also known that the signal transmission between neurons is disturbed. The nerve cells are hyper-excitable. As a result, they fire electrical impulses in a succession that could best be described as hectic. “This activity is somewhat reminiscent of epileptic activity. However, to date it was unclear how changes in cell morphology and abnormal function are related,” remarks Remy. “We have now found that if the form changes, this has a direct impact on the cell’s electrical properties. It’s just like in an electrical power cord. A thin cord that is also short has different electrical properties than a cord that’s thick and longer. We were able to show that the hyper-excitability can be explained by changes in the structure of the neurons.”
The neuroscientist emphasizes that this finding does not rule out other factors, such as alterations in cell metabolism. “However, our results demonstrate that the dysfunctions and the shape of the neurons are closely connected. Up until now we were not aware of this relationship.”
Precise Measurements and Computer Simulations
For their study, the scientists combined experimental research with computer simulations. At first, they examined the electrical activities of individual neurons as well as those of larger cell groups. For this purpose, they studied mice, whose brains exhibited Alzheimer-typical hallmarks. Furthermore, using high-precision microscope techniques, the scientists determined the dimensions of healthy and diseased nerve cells. Based upon this structural data, Remy’s team created a three-dimensional model of a single neuron and computed its electrical properties. In this way the researchers were able to relate cellular dysfunction to changes in cell morphology.
A General Effect
“Our study focused on Alzheimer’s. However, alterations in cell morphology are typical for all neurodegenerative diseases. Hence, we assume that the dysfunctions in cellular communication that manifest in other brain diseases are also resulting from structural changes. We think that this is a general effect shared by different diseases.”
In the opinion of the Bonn-based researcher, these findings cast a new light on pathological hallmarks. On the other hand, they could possibly also help with options for treatment. “Our results indicate that if one protects the structure of nerve cells, one also protects their functions. Pharmaceuticals aiming specifically at safeguarding the shape of neurons could potentially have a positive impact on disease progression. Cell morphology would be a novel approach for therapy,” says Remy. “Moreover, our computer model might prove helpful in studying the effects of these treatment options and in predicting their outcome.”
Story Source:
The above story is based on materials provided by DZNE – German Center for Neurodegenerative Diseases. Note: Materials may be edited for content and length.
Journal Reference:
- Zuzana Šišková, Daniel Justus, Hiroshi Kaneko, Detlef Friedrichs, Niklas Henneberg, Tatjana Beutel, Julika Pitsch, Susanne Schoch, Albert Becker, Heinz von der Kammer, Stefan Remy. Dendritic Structural Degeneration Is Functionally Linked to Cellular Hyperexcitability in a Mouse Model of Alzheimer’s Disease. Neuron, 2014; DOI: 10.1016/j.neuron.2014.10.024