Since the worst Ebola outbreak on record ignited last December in West Africa, scientists have been racing to develop drugs and vaccines to combat the virus. Several experimental drugs have been given to patients, and a new study details how scientists think one of those drugs might neutralize the virus.
The research focused on three antibodies, two of which are part of the experimental drug known as ZMapp. It hasn’t been officially tested in human clinical trials yet, but seven patients have received the drug so far this year. Though Ebola fatality rates sometimes approach 90 percent, five of the patients receiving ZMapp survived, including Americans Nancy Writebol and Kent Brantly. The drug has been shown to be incredibly efficient at treating Ebola infections in monkeys, all of which survived when treated. But it’s unknown how much the drug contributed to the human patients’ survival.
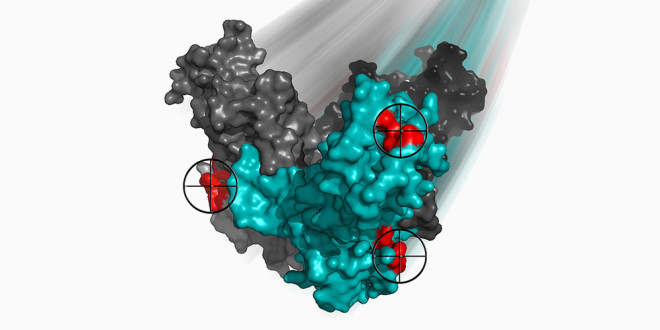
Antibodies in a drug that fights Ebola bind to three sites (red) on one of the viral proteins. Two of the antibodies lock the protein together and prevent the virus from invading host cells.
Jonathan AudetDesigning better drugs means understanding why the ones we have work, so a team of scientists recently took a close look at how some of the drug’s ingredients combat Ebola virus particles, and published the results today in Scientific Reports.
The trio of antibodies the scientists studied are part of a drug cocktail known as ZMAb, which has been shown to treat Ebola infections in mice, guinea pigs and monkeys.
Antibodies are proteins that recognize and respond to foreign substances, such as bacteria and viruses. ZMAb sends its three antibodies to find and knock out Ebola virus particles. In short, they interfere with the mechanism the virus uses to slip into host cells, keeping viral numbers low enough for the immune system to do its job.
All three antibodies target and bind to a protective protein the virus wears, called a glycoprotein. These glycoproteins protrude in spikes from the noodle-shaped Ebola particles and are essential both for infiltrating host cells and evading the host’s immune response.
The two of those three antibodies that are also used in the ZMapp drug, known as 2G4 and 4G7, adhere to sites near the base of the glycoprotein, near a border between two of the protein’s movable subunits. These antibodies appear to pin the protein together and prevent it from changing shape, which scientists think is a key maneuver during viral invasion of host cells.
“The antibodies are like molecular staples, or burly wrestlers pinning you down so you can’t move,” said Kartik Chandran, a virologist at Albert Einstein College of Medicine, who studies how Ebola virus gets into cells. “One of the virus’ Achilles heels is that they transform during infection. They’re like Swiss Army knives. Antibodies that can staple together two parts of the protein that move relative to each other are basically a good antiviral.”
Nancy Writebol, an American aid worker from North Carolina who was infected with the Ebola virus while working in Liberia, arrives at Emory University Hospital in Atlanta, Tuesday, Aug. 5, 2014.
The third antibody that isn’t part of the ZMapp combo doesn’t appear to be as potent a neutralizer as the first two. But scientists think it might be playing a role in attracting immune cells to the pinned virus. “Those could destroy viruses or infected cells,” said Erica Ollmann Saphire, a virologist at The Scripps Research Institute.
The precise mechanics of the antiviral onslaught aren’t totally worked out, but it’s clear that a combination of three antibodies works better than any single antibody on its own. One explanation, perhaps, is that it’s tricky for a virus to mutate and escape the activity of three antibodies at the same time, and that mutating and eluding just one is much easier.
That’s one of the reasons scientists often mix antibodies into cocktails, as they’ve done with ZMapp. The antibodies in that cocktail have been modified slightly for use in humans (see sidebar), but scientists don’t think that process (called “humanizing”) will change how the proteins interact with Ebola virus particles.
“What is the likelihood that the mouse and humanized antibodies work in the same way? That’s highly likely,” said viral immunologist James Crowe Jr. of Vanderbilt University, who is also working on developing Ebola antibodies.