Engineers at the University of California, San Diego, are proposing a new surgical intervention for children born with a single ventricle in their heart — instead of the usual two. The new approach would potentially reduce the number of surgeries the patients have to undergo in the first six months of life from two to just one. If successful, it would also create a more stable circuit for blood to flow from the heart to the lungs and the rest of the body within the first days and months of life.
Engineers ran computer simulations of the surgery and found it would reduce the workload on the patient’s heart by as much as half. It would also increase blood flow to the lungs and increase the amount of oxygen the body receives.
The surgery would introduce a radical change in the way infants with a single ventricle are treated. Currently, they undergo three surgeries by age three. Babies born with a single ventricle are severely deprived of oxygen, which makes their skin turn blue, and requires immediate medical intervention.
The research group, led by Alison Marsden, a professor of mechanical engineering, is working in collaboration with cardiothoracic surgeon Tain-Yen Hsia, of the Great Ormond Street Hospital for Children and UCL Institute of Cardiovascular Science in London. They reported their findings in an October issue of the Journal of Thoracic and Cardiovascular Surgery.
“Even when surgeries are successful, these babies live with a circulation that is very taxing on the one heart pump they have,” Dr. Hsia said. “So there is a need to find a better solution.”
The first surgery is performed in the first few days of life and installs a small GoreTex tube to connect the pulmonary arteries with a blood vessel off the aorta. That 3.5-millimeter shunt becomes the only source of blood to the infants’ lungs — essentially their only source of oxygen. There is a 30 percent mortality rate associated with this surgery.
Between 3 to 6 months, surgeons remove the shunt and connect the superior vena cava to the pulmonary artery. At that stage, half the blood flow needed for oxygenation goes through this circuit created by the physicians.
At around age 3, a third surgery, called a Fontan, connects both the inferior and superior vena cava to the pulmonary arteries, usually in a T-shaped configuration. Experience has shown that jumping directly to the second step too early in the child’s life, without allowing sufficient time for patients to grow, resulted in very high fatality rates for pediatric patients.
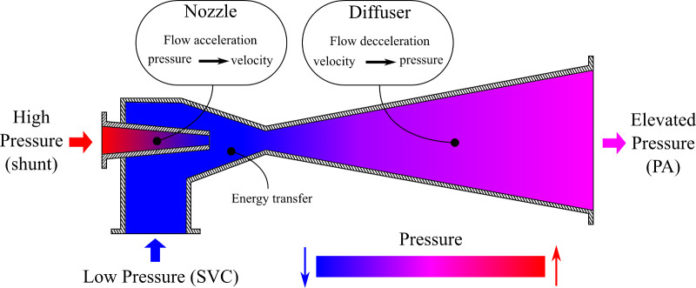
Engineers are proposing to combine the first and second steps of the surgery, with a small modification. They propose to have the shunt, slightly clipped, go into the superior vena cava, while also connecting the superior vena cava to the pulmonary arteries.
This would create what’s known in fluid mechanics as an ejector pump. The clipped shunt creates what’s called a Venturi effect, driving a low-pressure flow stream with an injection of a high-pressure flow stream and causing the speed of the blood flow to increase. “We feel it’s our job as engineers to propose ideas that represent out of the box thinking,” Marsden said. “This is a classic example of interdisciplinary translation of ideas from traditional areas of engineering that may help to improve patient care.” The work originated with Mahdi Esmaily-Moghadam, who was a Ph.D. student in Marsden’s lab and now is a postdoctoral researcher at Stanford University.
The shunt could be closed later, when circulation improves, via a catheter — a much less invasive procedure. Researchers call the proposed surgery an assisted bidirectional Glenn. They are working with experimentalists at Clemson University, who have built a silicon model to simulate the surgery in the lab. Preliminary results are encouraging. The next step will likely be to try this surgery on a sheep model.
Marsden and her group are no strangers to proposing new designs for pediatric heart surgery. In 2009, she and colleagues proposed a custom-made Y-shaped design for the Fontan surgery, rather than the traditional T-shaped connection used. In 2010-11, six patients underwent a Y-graft surgery at Stanford University.
In March and September of this year, Marsden and her co-authors at Stanford published two papers evaluating the results of the surgeries. All six patients were alive and doing well, given their condition. One patient had developed a blood clot in one of the branches of the Y-graft, but that has not impacted the child’s health. Researchers also found that the Y-graft reduced energy losses in the blood flow and distributed blood flow more evenly to both lungs.
To create simulations, Marsden’s team uses SimVascular (simvascular.org). Her group and a group of researchers at UC Berkeley received a $1 million grant from the National Science Foundation to make the software truly open source and accessible to researchers and physicians all over the world.
“It’s the only open-source software that gives researchers a full pipeline to go from patients’ medical imaging to a 3D model of vasculature that allows researchers to analyze and predict sheer stress and wall deformation through [a computational method called] finite element analysis,” Marsden said. Marsden and her colleagues hope that SimVascular may be used in the future to impact a wide range of cardiovascular surgeries and devices in children and adults.
Story Source:
The above story is based on materials provided by University of California – San Diego. Note: Materials may be edited for content and length.
Journal Reference:
- Mary Hunt Martin, Jeffrey A. Feinstein, Frandics P. Chan, Alison L. Marsden, Weiguang Yang, V. Mohan Reddy. Technical feasibility and intermediate outcomes of using a handcrafted, area-preserving, bifurcated Y-graft modification of the Fontan procedure. The Journal of Thoracic and Cardiovascular Surgery, 2014; DOI: 10.1016/j.jtcvs.2014.08.058