Hematopoietic stem cells (HSCs) give rise to all blood and immune cells throughout the life of vertebrate organisms, from zebrafish to humans. But details of their genesis remain elusive, hindering efforts to develop induced pluripotent stem cell (iPSC) replacements that might address a host of blood disorders.
In a paper published Nov. 20 in the journal Cell, researchers at the University of California, San Diego School of Medicine describe the surprising and crucial involvement of a pro-inflammatory signaling protein in the creation of HSCs during embryonic development, a finding that could help scientists to finally reproduce HSCs for therapeutic use.
“The recent breakthrough of induced pluripotency has made the concept of patient-specific regenerative medicine a reality,” said principal investigator David Traver, PhD, professor in the Department of Cellular and Molecular Medicine. “The development of some mature cell lineages from iPSCs, such as cardiac and neural, has been reasonably straightforward, but not with HSCs. This is likely due, at least in part, to not fully understanding all of the factors used by the embryo to generate HSCs. We believe the discovery that pro-inflammatory cues are important in vivo will help us recapitulate instruction of HSC fate in vitro from iPSCs.”
Traver and colleagues specifically looked at the role of a cytokine (a type of cell signaling protein) called tumor necrosis factor alpha or TNFa, which plays a pivotal role in regulating systemic inflammation and immunity. The work extended previous research by Spanish biologist Victoriano Mulero, who had reported that TNFa was important in the function of the embryonic vascular system and that in animal models where TNF function was absent, blood defects resulted.
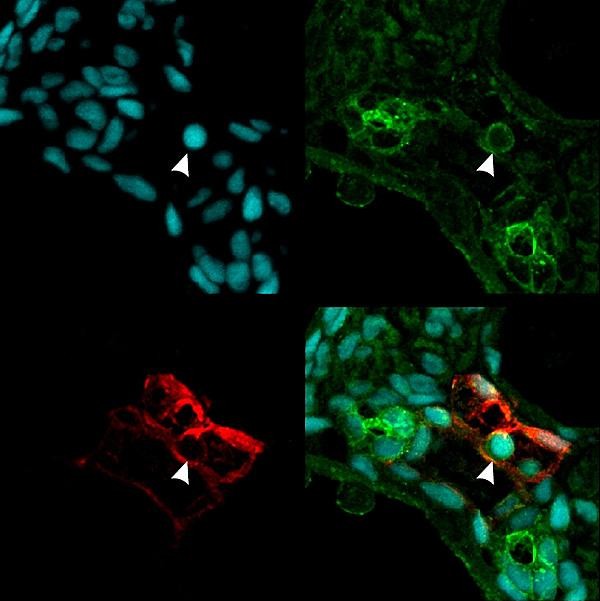
The Cell paper’s first author Raquel Espin-Palazon, a postdoctoral researcher in Traver’s lab and a former colleague of Mulero’s, determined that TNFa was required for the emergence of hematopoietic stem cells during embryogenesis in zebrafish — a common animal model.
Traver said the finding was completely unexpected because HSCs emerge relatively early in embryonic formation when the developing organism is considered to be largely sterile and devoid of infection.
“Thus, there was no expectation that pro-inflammatory signaling would be active at this time or in the blood-forming regions,” Traver said. “Equally surprising, we found that a population of embryonic myeloid cells, which are transient cells produced before HSCs arise, are the producers of the TNFa needed to establish HSC fate. So it turns out that a small subset of myeloid cells that persist for only a few days in development are necessary to help generate the lineal precursors of the entire adult blood-forming system.”
The newly discovered role of TNFa in HSC development mirrors a parallel discovery regarding interferon gamma (INFg), another cytokine and major mediator of pro-inflammatory signaling, highlighting multiple inputs for inflammatory signaling in HSC emergence. Traver said the crucial roles of TNFa and INFg in HSC emergence are likely similar in humans because of the highly conserved nature of HSC development across vertebrate evolution.
Story Source:
The above story is based on materials provided by University of California, San Diego Health Sciences. Note: Materials may be edited for content and length.