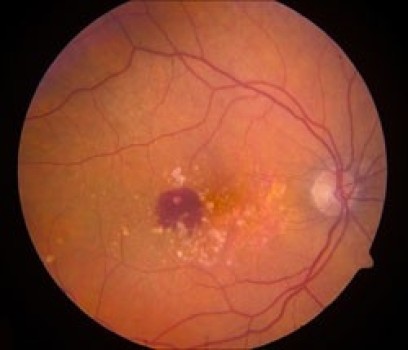
Stanford University School of Medicine scientists have found a new way to forecast which patients with age-related macular degeneration are likely to suffer from the most debilitating form of the disease.
The new method predicts, on a personalized basis, which patients’ AMD would, if untreated, probably make them blind, and roughly when this would occur. Simply by crunching imaging data that is already commonly collected in eye doctors’ offices, ophthalmologists could make smarter decisions about when to schedule an individual patient’s next office visit in order to optimize the chances of detecting AMD progression before it causes blindness.
AMD is the leading cause of blindness and central vision loss among adults older than 65. An estimated 10-15 million people in the United States suffer from the disease, in which the macula — the key area of the retina responsible for vision — shows signs of degeneration. During normal aging, yellowish deposits called drusen form in the retina, which is the light-sensitive layer of tissue at the back of the eye. As drusen increase in size and number, they eventually begin to damage the light-sensitive cells of the macula. This stage of the disease, called “dry” AMD, can mean blurry central vision and impaired day-to-day activity.
While about four of every five people with AMD have the dry form of the disease, it’s the so-called “wet” form that most concerns ophthalmologists, because it accounts for 80-90 percent of all legal blindness associated with the disease. In wet AMD, abnormal blood vessels accumulate underneath the macula and leak blood and fluid. When that happens, irreversible damage to the macula can quickly ensue if not treated quickly.
But until now, there has been no effective way to tell which individuals with AMD are likely to progress to the wet stage. Current treatments are costly and invasive — they typically involve injections of medicines directly into the eyeball — making the notion of treating people with early or intermediate stages of AMD a non-starter. Doctors and patients have to hope the next office visit will be early enough to catch wet AMD at its onset, before it takes too great a toll.
Predicting Progression to ‘Wet’ AMD
In a study published in the November issue of Investigative Ophthalmology & Visual Science, the researchers derived a formula that they say predicts, with high accuracy, whether a patient with mild or intermediate AMD will progress to the wet stage. The formula distinguishes likely from unlikely progressors by analyzing patient data that’s routinely collected by ophthalmologists and optometrists when they perform retinal scans with an imaging technique called spectral domain optical coherence tomography.
This imaging technique is analogous to ultrasound: The macula is scanned with a beam of focused laser light, and the amount of reflected light coming back at each point is measured and recorded. The resulting stream of data is computationally converted into an extremely high-resolution, three-dimensional image.
“Right now, a patient who goes into the ophthalmologist’s office typically gets an SD-OCT scan anyway,” said the study’s senior author, Daniel Rubin, MD, assistant professor of radiology and of biomedical informatics. “Our technique involves no new procedures in the doctor’s office — patients get the same care they’ve been getting anyway. We’ve simply added on a computerized image-processing step that analyzes not only that scan but any previous ones available from that same patient’s earlier visits.”
Generating a Risk Score
From this computerized analysis, the investigators are able to generate a risk score: a number that predicts a patient’s likelihood of progressing to the wet stage within one year, three years or five years. The likelihood of progression within one year is most relevant, because it translates into a concrete recommendation: how soon to schedule the patient’s next office visit.
Until now, attempts to predict AMD progression have relied on eye doctors examining color photographs of the retina taken in their offices. There is no way to translate that information into risk scores. The high-resolution imaging technique, Rubin said, provides much richer detail. “You can almost see individual cells,” he said. Plus, it is far more amenable to digital analysis. Previously proposed predictive models have shown some accuracy over long periods of time, but none has been adequately accurate over the shorter, one-year time frame that’s relevant to making decisions about office-visit frequency, Rubin said.
In the study, the Stanford team analyzed data from 2,146 scans of 330 eyes in 244 patients seen at Stanford Health Care over a five-year period. They found that certain key features in the images, such as the area and height of drusen, the amount of reflectivity at the macular surface and the degree of change in these features over time, could be weighted to generate a patient’s risk score. Patients were followed for as long as four years, and predictions of the model were compared with actual instances of progression to wet AMD. The model accurately predicted every occurrence of progression to the wet stage within a year. About 40 percent of the time when the model did predict progression to wet AMD within a year, the prediction was not borne out.
“No test gets it right 100 percent of the time,” Rubin said. “You can tweak the model to trade off the risk of telling someone they will progress when they actually won’t against the risk of telling them they won’t progress when they actually will. With AMD you really don’t want any false negatives, so you tune the model accordingly. The downside is that some patients will wind up being told to come in sooner than, in fact, they probably need to. But that’s nothing compared with the downside of a patient at high risk for progression’s not coming in soon enough.”
Larger Studies Needed
Rubin emphasized that this proof-of-principle study needs to be followed up by a larger study, ideally using data gathered from patients seen at other institutions. He and his associates have now embarked on such a study.
Story Source:
The above story is based on materials provided by Stanford University Medical Center. Note: Materials may be edited for content and length.
Journal Reference:
- L. de Sisternes, N. Simon, R. Tibshirani, T. Leng, D. L. Rubin. Quantitative SD-OCT Imaging Biomarkers as Indicators of Age-Related Macular Degeneration Progression. Investigative Ophthalmology & Visual Science, 2014; DOI: 10.1167/iovs.14-14918