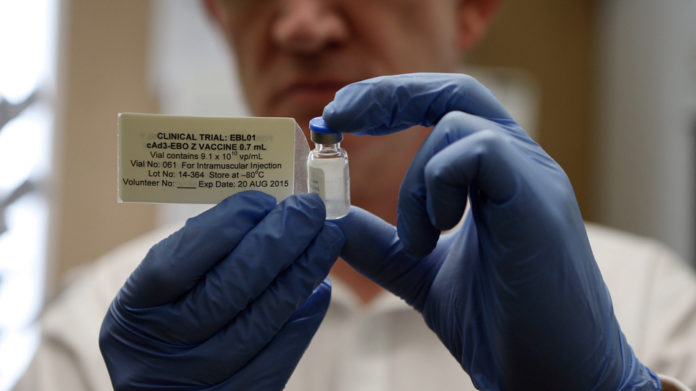
The 5-step process for making an Ebola vaccine

Jonas Salk, inventor of the polio vaccine, was born 100 years ago this week, and the contributions he made to science still save countless lives. That’s because the scientific dogma behind his vaccine still holds true: If you expose a body to deactivated, noncontagious version of a virus, when a live bug comes along, that body will be ready.
The same principle would apply to an Ebola vaccine. That is, if one was available for the human body. Ebola vaccines exist, but until now, they have only been tested in monkeys.
Human clinical trials for an Ebola vaccine began only this year. The World Health Organization reports the data from these first tests will be available in December.
“We need to speed up that time point,” says Clive Gray, a professor of immunology at the University of Cape Town in South Africa. By December, the efforts may be too little, too late. And results from the first phase of trials don’t mean wide-scale production. They mean more clinical trials. The time clock is grim and running thin: The most pessimistic scenarios predict as many as 1.4 million cases of the disease by January.
It’s not the science that has held an Ebola vaccine back. In 2005, researchers reported a vaccine that was 100% effective in protecting monkeys from the illness. That should have promoted a human trial. But it didn’t.
Why? Simply and sadly, it was bad business for drug manufacturers. Past outbreaks have been small and confined to poor countries with faltering public-health systems. Now, with international attention focused on Ebola, an Ebola vaccine will become a reality. WHO reports that clinical trials are underway in three countries and will soon commence in four others.
But the crux of the Ebola-vaccine problem is this: It takes time to take a vaccine that’s effective in monkeys and ensure its safety and efficacy in humans. “As we accelerate in a matter of weeks a process that typically takes years, we are ensuring that safety remains the top priority, with production speed and capacity a close second,” Marie-Paule Kieny, WHO assistant director-general, said in a press release.
So what does it take to bring a vaccine from the lab to the field where it is desperately needed? I asked Dr. Gray to lay out how it would work. To simplify, it takes five steps. And we’re only on Step 3.
Step 1: Identify the target
The first step in creating a vaccine is to study the structure of the virus. Knowing the structure allows scientists to mimic its structure in a serum. Again, that’s the key to an effective vaccine: to inject into somebody a substance that looks like the virus, but is not the virus.
This process is a lot harder for some viruses than others. HIV and the flu, for instance, are constantly mutating, creating new forms that can fool the immune system. That’s why a new flu strain crops up every year. HIV is even more elusive — it can mutate and evade an immune response within one person’s body.
Thankfully, Ebola isn’t like the flu or HIV. “The current Ebola outbreak is the same viral strain — the Zaire Ebola virus strain — as the very first outbreak,” Gray says. “And it hasn’t changed. Each person who becomes infected, gets infected with the same virus.” That means one vaccine can protect all people from Ebola, without the need for yearly updates.
Step 2: Mimic the target
All the immune system needs to identify a virus is the virus’s outer protein coat. The outer protein coat is the virus’s calling card. That’s what scientists have recreated in the Ebola vaccines now in trial. “They are taking the genetic material from Ebola that codes for the protein coat and they insert that genetic material into the skeleton of another virus,” Gray says.
That skeleton virus won’t get a person sick, but will, in a sense, tell the immune system about the Ebola virus. The body will then start producing the antibodies necessary to contain Ebola.
Step 3: Test in animals
The animal tests ensure that the vaccine provokes the intended immune response against the Ebola virus. “The animal testing has been done on these existing vaccines,” Gray says. “That’s where the field is at now.”
Up until this point, The New York Times explains, “the research may have cost a few million dollars, but tests in humans and scaling up production can cost hundreds of millions, and bringing a new vaccine all the way to market typically costs $1 billion to $1.5 billion.” And that’s why we’ve stalled at Step 3.
Step 4: Test in humans
That animal-tested vaccine would not give any human Ebola, but that doesn’t mean it’s safe for humans. Early-stage vaccines can have dangerous side effects.
In the very first clinical trials, researchers “are putting these vaccines into people to make sure they are safe, and also to see if the immune response in the people looks very similar to the immune response in the monkeys in the animal studies,” Gray says. Once it is determined to be safe and effective, the correct dosage needs to be assessed. “If you give too much, maybe it would make the person feel sick, and if you give too little, it won’t be effective,” he says.
Researchers will also consider the mode of injection — should it be injected into the muscle or just under the skin?
These clinical trials need to be conducted carefully, and can often take years to ensure a vaccine that is safe for mass consumption. “You cannot give people something that is unsafe,” Gray says.
Step 5: Produce and disperse
What does this step involve? “Funding, simple as that,” Gray says. “If you give enough money, if you give enough dollars, it can be scaled up quickly.” GlaxoSmithKline, the pharmaceutical mega-giant, has estimated it could produce 1 million doses of a vaccine a month by 2016. Two years might be lightening fast when it comes to drug approvals, but when looking at the trajectory of the current Ebola outbreak, two years may be an eternity.
- Republican 2016 Contenders Have Taken Positions on NSA Reform. Where Does Hillary Clinton Stand?
- The Feds Want to Redefine TV, and That Has Cable Giants Nervous
- Should Schools Mandate Computer-Coding Classes?
- Commuting Soothsayers Look for Alternatives to Driving
This article originally published at National Journal here