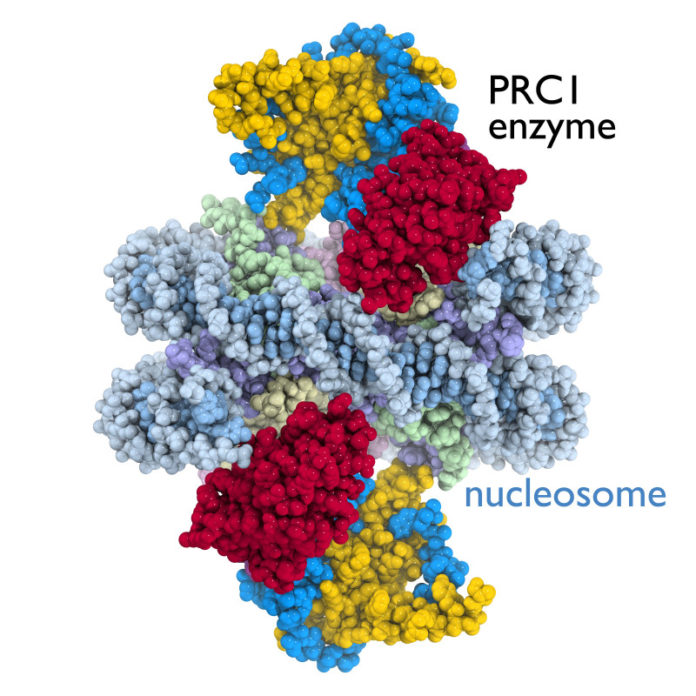
A landmark study to be published in the October 30, 2014 print edition of the journal Nature provides new insight into the function of an enzyme related to the BRCA1 breast cancer protein. The study by a team at Penn State University is the first to produce a detailed working image of an enzyme in the Polycomb Repressive Complex 1 (PRC1) — a group that regulates cell development and is associated with many types of cancer.
Enzymes like PRC1 turn on or turn off the activity of genes in a cell by manipulating individual chromosome units called nucleosomes. “The nucleosome is a key target of the enzymes that conduct genetic processes critical for life,” said Song Tan, professor of biochemistry and molecular biology at Penn State University and the leader of the study’s research team.
The Penn State scientists obtained the first crystal structure of a gene regulation enzyme while it is working on a nucleosome. The image reveals previously unknown information about how the enzyme attaches to its nucleosome target. Before this study, scientists had been unable to picture exactly how cancer-related enzymes in the PRC1 group interacted with a nucleosome to control gene activity. The study is also the first to determine the crystal structure of a multisubunit protein complex bound to a nucleosome, which itself is a complex assembly of DNA and 4 histone proteins.
The research is the culmination of over 12 years of research by the Tan laboratory to capture an image of this important class of enzymes bound to the nucleosome. His lab earlier had determined the first structure of another nucleosome-bound protein, RCC1. “This is the second important structure from the Tan lab to date of a nucleosome in complex with a protein known to interact with and modify chromatin behavior, which in turn can influence human gene expression,” said Peter Preusch, Ph.D., of the National Institutes of Health’s National Institute of General Medical Sciences, which partially funded the research. “Along with Dr. Tan’s previous work detailing a nucleosome bound to the key regulatory protein, RCC1, this new structure adds to our knowledge of how proteins can regulate the structure and function of our genetic material.”
The research project was proposed and executed by team member Robert K. McGinty, a Damon Runyon postdoctoral fellow at Penn State. McGinty and Ryan C. Henrici, an undergraduate in the Penn State Schreyer Honors College, grew crystals of the PRC1 enzyme bound to the nucleosome. The team then solved the three-dimensional structure of this large molecular assembly by X-ray crystallography. “We are excited about this crystal structure because it provides new paradigms for understanding how chromatin enzymes function,” McGinty said.
The study performed in the Penn State Center for Eukaryotic Gene Regulation provides unexpected insight into the workings of the BRCA1 breast-cancer-associated tumor-suppressor protein. Like PRC1, BRCA1 is a chromatin enzyme that shares a similar activity on the nucleosome. Tan said, “Our study suggests that BRCA1 and PRC1 employ a similar mechanism to anchor to the nucleosome.” Tan and his team now are working to visualize how BRCA1 and other disease-related chromatin enzymes interact with the nucleosome.
Story Source:
The above story is based on materials provided by Penn State. Note: Materials may be edited for content and length.
Journal Reference:
- Robert K. McGinty, Ryan C. Henrici, Song Tan. Crystal structure of the PRC1 ubiquitylation module bound to the nucleosome. Nature, 2014; 514 (7524): 591 DOI: 10.1038/nature13890