Cells can huddle to communicate within a restricted group, scientists at EMBL Heidelberg have found. The study is the first demonstration that the way cells organise themselves influences their ability to communicate. The researchers propose that this strategy, which they discovered in developing zebrafish, could be much more widespread, influencing processes like wound repair, organ formation and even cancer.
From basketball to handball, rugby to American football, teams in a variety of sports huddle together to agree tactics in secret. Cells, too, can huddle to communicate within a restricted group, scientists at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, have found. The study, published today in Nature, is the first demonstration that the way cells organise themselves influences their ability to communicate. The researchers propose that this strategy, which they discovered in developing zebrafish, could be much more widespread, influencing processes like wound repair, organ formation and even cancer.
“Everybody can speak, everybody can listen, but what’s said in the group stays in the group,” explains Sevi Durdu, who carried out the research, “by huddling together, these cells trap and concentrate a signal to communicate only amongst themselves.”
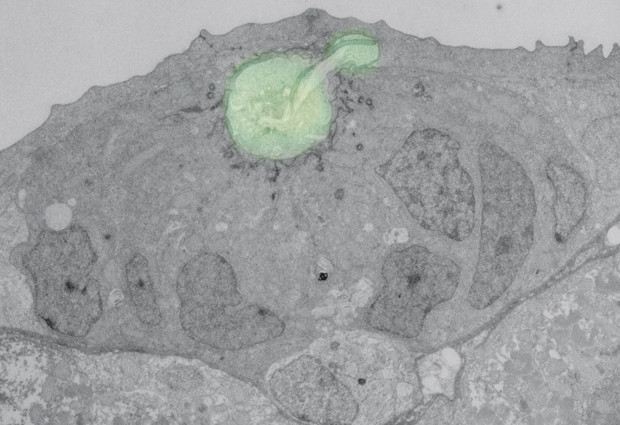
Durdu, a PhD student in Darren Gilmour’s lab at EMBL, found this behaviour in specific groups of cells in the zebrafish: the cells that will develop into the animal’s ‘lateral line’, a series of ear-like organs along the fish’s flank that allow it to sense changes in water pressure. As a zebrafish develops, a mass of cells moves along the developing animal’s side. At the point where one of these organs should form, a group of cells at the rear assembles into a huddle and stops, eventually developing into the organ. The rest of the cells, meanwhile, have moved on, until another group stops to form another organ, and so on. The cells that group together and stop to form the future organ also change shape, going from flat, crawling cells to upright, tear-shaped cells that come together like cloves in a bulb of garlic. Durdu found that these ‘garlic cloves’ huddle around a shared space, or lumen, in which they trap a molecule cells use to communicate: FGF.
“Normally, FGF acts as a long-range communication signal. In the lateral line, we find that most of this signal is normally just wafting over the cells’ heads,” says Gilmour. “But when cells get together and huddle they can trap and concentrate this signal in their shared lumen, and make a decision that the others can’t: they stop moving.”
The EMBL scientists found that, by enabling a group of cells to increase the concentration of FGF they are in contact with, the shared lumen plays a critical role in determining when and where the huddles stop moving. When the scientists increased the concentration of FGF, cell huddles came to a standstill more abruptly, forming organs that were closer together. And when they decreased the level of FGF, huddles continued to migrate for longer and formed organs that were further apart.
“All epithelial cells — and that’s the cells that make up most of the organs in our bodies — can do this, so you could imagine that this type of local chamber could be forming transiently in many different parts of the body, whenever cells need to self-organise and communicate,” Gilmour says.
When the scientists broke up cell huddles in their zebrafish embryos, FGF leaked out. When this happens the cells in a group are no longer able to communicate efficiently, leading the scientists to wonder if this influence of organisation on communication could play a role in wound repair. When our skin is scratched, cells that were standing upright ‘lie down’ and start crawling — in essence, local huddles break up and cells change their behaviour. Another situation where cells may be huddling to communicate within a group, Gilmour and Durdu posit, is in organoids — self-assembled organ-like structures grown in the lab, which start by forming a common lumen.
In future, Gilmour and colleagues would like to understand the interplay between the ability — or decision — to stop and signals that they previously found drive cells to move forward, and how both are influenced by changes in cell shape.
Story Source:
The above story is based on materials provided by European Molecular Biology Laboratory (EMBL). Note: Materials may be edited for content and length.
Journal Reference:
- Sevi Durdu, Murat Iskar, Celine Revenu, Nicole Schieber, Andreas Kunze, Peer Bork, Yannick Schwab, Darren Gilmour. Luminal signalling links cell communication to tissue architecture during organogenesis. Nature, 2014; DOI: 10.1038/nature13852