Two listeners might hear the same message, but understand it differently and take different actions in response. Something similar happens within the hair follicle: Stem cells and their progeny react quite differently to an important group of signaling proteins.
New experiments at Rockefeller University have given researchers a better handle on how this happens. The results, published online October 9 in Cell Stem Cell, offer new insights into the regulation of stem cells and the process that leads to fully differentiated cells.
“Both hair follicle stem cells and a set of transitional progenitors that arise from them respond to growth factors called BMPs,” says Elaine Fuchs, Rebecca C. Lancefield Professor and head of the Robin Chemers Neustein Laboratory of Mammalian Cell Biology and Development. “We wanted to know whether stem cells and their progeny interpret the signal differently and if so how. By examining how stem cells change their interpretations of signals as they embark along the path to making tissue, we can gain a better general understanding of how stem cells are able to both renew themselves and also make and repair tissues, as well as how disrupted signaling might lead to diseases, such as cancer.”
“Nearly every tissue of our body has resident stem cells, whose job is to replace dying tissue cells and repair wounds after injury,” explains Fuchs. “In many tissues, stem cells divide infrequently but must be called into action rapidly to generate tissue in response to injury.”
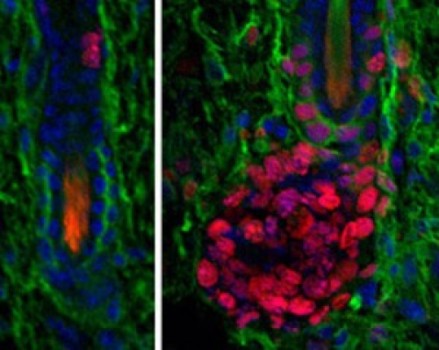
Hair follicles cycle through bouts of rest, growth and destruction, she says, making these tiny organs ideal subjects for studying how stem cell behavior changes as they make tissue. Once activated, many stem cells, including those of the hair follicle, produce short-lived progeny, called transiently amplifying cells (TACs), an intermediate step on the way to producing differentiated tissue cells. In the hair follicle, TACs give rise to the specific types of cells that form the hair shaft and the channel that supports it.
Previously, the Fuchs lab discovered that BMPs help keep hair follicle stem cells in their resting phase. When BMP signaling is turned off in these stem cells, the stem cells begin to proliferate and make TACs. BMPs are believed to then play a subsequent role in telling TACs to specialize and differentiate into the hair shaft or the channel.
“In separate experiments, we disabled the receptors that bind to BMPs in hair follicle stem cells and in TACs,” says Maria Genander, the first author and a former postdoc in the lab, who is now with the Karolinska Institutet in Sweden. “For the stem cells, we confirmed previous results: BMP signaling helps prevent stem cells from dividing, and when it is lost irreversibly, this can result in tumors. With regard to the TACs, we discovered that loss of BMP signaling causes these cells to produce an excess of supportive channel cells at the expense of hair shaft progenitors.”
Once a cell receives a BMP signal, it activates a DNA transcription factor of the SMAD family. In the nucleus, activated SMAD complexes bind to DNA and change the expression of genes that affect how the cells behave.
To better understand the mechanics behind BMPs’ distinct roles in stem cells and TACs, Genander looked at the locations on the cells’ genome where SMADs bound. “We discovered that despite many similarities, SMADs also bound to unique sets of genes in stem cells versus TACs, and each non-overlapping set correlated to the tailor-made jobs that these cells have in the hair follicle,” Genander says. She and her co-authors then confirmed these gene roles in stem cell quiescence and hair production by inactivating them in mice.
“There are still many mysteries to be solved,” Fuchs says. “These results gave us some clues as to how SMADs might selectively bind to one set of target genes in stem cells and another in its progeny, but there is much left to learn about how the sequential process of target gene selection and differentiation is controlled and how it changes along the path to tissue production. By examining the in vivo mechanics of BMP signaling in stem cells and their progeny within their native tissue, we have begun to understand how the same signal can influence not only what the stem cells do, but also what their progeny do, and with remarkably distinct outcomes. It will be interesting to see if these paradigms hold in other adult stem cells of the body, and how this goes awry in cancers.”
Story Source:
The above story is based on materials provided by Rockefeller University. Note: Materials may be edited for content and length.
Journal Reference:
- Maria Genander, Peter J. Cook, Daniel Ramsköld, Brice E. Keyes, Aaron F. Mertz, Rickard Sandberg, Elaine Fuchs. BMP Signaling and Its pSMAD1/5 Target Genes Differentially Regulate Hair Follicle Stem Cell Lineages. Cell Stem Cell, 2014; DOI: 10.1016/j.stem.2014.09.009