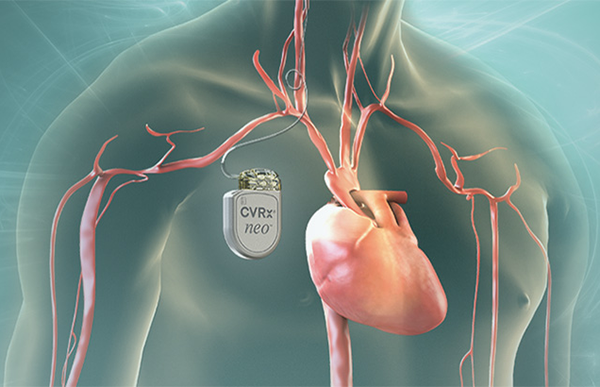
CVRx, a company out of Minneapolis, Minnesota, received CE Mark approval for its Barostim neo System that’s intended for heart failure patients with an “ejection fraction ≤ 35% and a New York Heart Failure Classification of III without restriction on QRS duration, concomitant medical device treatment or presence of atrial fibrillation,” according to the announcement. The device had received previous European approval for use on patients with drug resistant hypertension.
 The Barostim neo is an electrical stimulator with a lead going up to the carotid artery where it excites baroreceptors that in turn regulate cardiovascular activity. It continuously adjusts the signals it delivers, aiming to provide an optimal beat-to-beat stimulation. The device can be turned on and off following implantation and is programmable for individual patient’s unique cardiac health. .
The Barostim neo is an electrical stimulator with a lead going up to the carotid artery where it excites baroreceptors that in turn regulate cardiovascular activity. It continuously adjusts the signals it delivers, aiming to provide an optimal beat-to-beat stimulation. The device can be turned on and off following implantation and is programmable for individual patient’s unique cardiac health. .
Some of the data that led to the approval:
CVRx completed enrollment of a 140 patient randomized, controlled clinical trial to determine the performance of Barostim Therapy for patients suffering from chronic heart failure with advanced symptoms. Promising results from an earlier study demonstrating clinical improvement and reduced hospitalizations have been presented and published. The six month results from the randomized, controlled trial are being prepared for publication.
Five-year results of the 322-patient sham-controlled Rheos Hypertension Trial were presented at the American Society of Hypertension and the joint European and International Societies of Hypertension annual scientific conferences in May and June of 2014. The results showed a statistically significant reduction of systolic and diastolic blood pressure in excess of 32mmHg and 17mmHg, respectively, over the course of 5 years. In addition, the long term safety profile of the therapy proved to be excellent with very low rates of stroke, myocardial infarction and worsening of carotid stenosis in this population of patients with advanced hypertension.
Press release: CVRx® Receives CE Mark Approval of the Barostim neo System™ for the Treatment of Heart Failure…
Product page: Barostim neo…