In the race to find more effective ways to treat cancer, Boise State University biophysicist Daniel Fologea is working outside the rules of general mathematics that say one plus one equals two. In his world, one plus one adds up to a whole lot more.
While radiotherapy can precisely target just the tumor site, systemic chemotherapy spreads a wide net, sending drugs speeding throughout the entire body in an attempt to kill cancer cells while also killing many healthy cells. Neither of these methods is highly effective when applied alone, therefore separated sessions of chemo and radiotherapy are required when fighting against solid tumors.
Reports have shown that ideally, both methods would be employed at the same time. But doing so produces levels of toxicity that often are deadly. To reduce the remote toxicity inherent to chemotherapy, the drugs can be administered into solid tumors by using liposomes, which are nanoscale vesicles made from fats and loaded with anti-cancer drugs. Liposomes self-accumulate within the tumor but the loaded drugs will be released very slowly from their encasing.
A new patent awarded to Fologea, a professor in the Department of Physics, and co-researchers from the University of Arkansas in August 2014 holds promise of a way to combine the oomph of chemotherapy with the precision of radiotherapy, without harm to healthy cells.
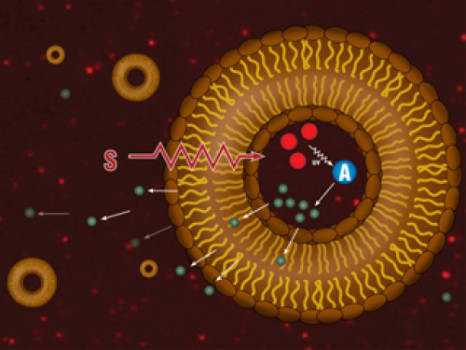
In the new approach, said Fologea, “The liposomes are designed to release their precious cargo upon exposure to x-ray. Not only does this target where the medication goes, it also allows for a huge concentration of the drug to be released at once at the tumor site, thus increasing its efficacy. In addition, this combined modality of treatment employing concomitant radio and chemotherapy is supra-additive, which means it is several times more efficient than each therapy applied alone.”
Here’s how it works: liposomes have small scintillating nanoparticles embedded within them. When hit with the x-ray, they emit ultraviolet (UV) light. UV light triggers the release of Ca2+ entrapped into a photolabile cage inside the liposomes. The free Ca2+ activates an enzyme called phospholipase A2 that starts chewing the fats in the wall of the liposomes and triggers the fast release of the drug.
Now that they have a patent on the technique, researchers still expect several years of testing before the method is approved and available for cancer patients.
In the meantime, Fologea completed the initial phase of another method to provide similar results by using only materials previously approved by the FDA for treatment of cancer and other diseases. This approach will pave the way for earlier translational studies.
Working with Boise State biology professor Cheryl Jorcyk, he is looking for ways to put antibodies on the surface of the liposome, allowing them to recognize and attack cancer cells that are circulating in the body. A distinct approach to develop liposomes useful for treatment of diabetes is under development with Boise State biology professor Denise Wingett.
Story Source:
The above story is based on materials provided by Boise State University. The original article was written by Kathleen Tuck. Note: Materials may be edited for content and length.