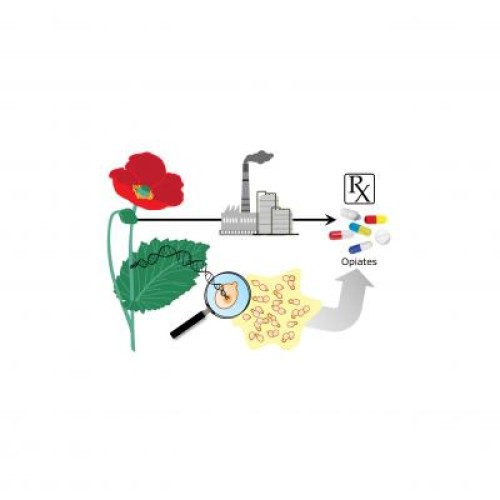
For centuries poppy plants have been grown to provide opium, the compound from which morphine and other important medicines such as oxycodone are derived.
Now bioengineers at Stanford have hacked the DNA of yeast, reprograming these simple cells to make opioid-based medicines via a sophisticated extension of the basic brewing process that makes beer.
Led by Associate Professor of Bioengineering Christina Smolke, the Stanford team has already spent a decade genetically engineering yeast cells to reproduce the biochemistry of poppies with the ultimate goal of producing opium-based medicines, from start to finish, in fermentation vats.
“We are now very close to replicating the entire opioid production process in a way that eliminates the need to grow poppies, allowing us to reliably manufacture essential medicines while mitigating the potential for diversion to illegal use,” said Smolke, who outlines her work in the August 24th edition of Nature Chemical Biology.
In the new report Smolke and her collaborators, Kate Thodey, a post-doctoral scholar in bioengineering, and Stephanie Galanie, a doctoral student in chemistry, detail how they added five genes from two different organisms to yeast cells. Three of these genes came from the poppy itself, and the others from a bacterium that lives on poppy plant stalks.
This multi-species gene mashup was required to turn yeast into cellular factories that replicate two, now-separate processes: how nature produces opium in poppies, and then how pharmacologists use chemical processes to further refine opium derivatives into modern opioid drugs such as hydrocodone.
From Plants to Pills Today
Plant-derived opium has been used and abused for centuries, but a good place to begin the modern story is with the use of morphine during World War II.
Morphine is one of three principal pain killers derived from opium. As a class they are called opiates. The other two important opiates are codeine, which has been used as a cough remedy, and thebaine, which is further refined by chemical processes to create higher-value therapeutics such as oxycodone and hydrocodone, better known by brand names such as OxyContin and Vicodin, respectively.
Today legal poppy farming is restricted to a few countries–including Australia, France, Hungary, India, Spain and Turkey–supervised by the International Narcotics Control Board, which seeks to prevent opiates like morphine, for instance, from being refined into illegal heroin.
The biggest market for legal opiates, and their opioid derivatives, is the United States, where pharmaceutical factories use chemical processes to create the refined products that are used as pain-killing pills. However poppies are not grown in significant quantities in the U.S., creating various international dependencies and vulnerabilities in the supply of these important medicines.
Turning Yeast Into a Pharmaceutical Factory
The thrust of Smolke’s work for a decade has been to pack the entire production chain, from the fields of poppies, through all the subsequent steps of chemical refining, into yeast cells using the tools of bioengineering.
What Smolke’s team has now done is to carefully reprogram the yeast genome — the master instruction set that tells every organism how to live — to behave like a poppy when it comes to making opiates.
The process involved more than simply adding new genes into yeast. Opioid molecules are complex three-dimensional objects. In nature they are made in specific regions inside the poppy. Since yeast cells do not have these complex structures and tissues, the Stanford team had to recreate the equivalent of poppy-like “chemical neighborhoods” inside their bioengineered yeast cells.
It takes about 17 separate chemical steps to make the opioid compounds used in pills. Some of these steps occur naturally in poppies and the remaining via synthetic chemical processes in factories. Smolke’s team wanted all the steps to happen inside yeast cells within a single vat, including using yeast to carry out chemical processes that poppies never evolved to perform — such as refining opiates like thebaine into more valuable semi-synthetic opioids like oxycodone.
So Smolke programmed her bioengineered yeast to perform these final industrial steps as well. To do this she endowed the yeast with genes from a bacterium that feeds on dead poppy stalks. Since they wanted to produce several different opioids, the team hacked the yeast genome in slightly different ways to produce each of the slightly different opioid formulations, such as oxycodone or hydrocodone.
The Missing Link
All of this was demonstrated in the new paper. But Smolke’s team must still clear one more hurdle in order to achieve the goal of pouring sugar into a stainless steel vat of bioengineered yeast and skimming off specific opioids at the end of the process. They must perform another set of bioengineering hacks to connect the two major advances they have made over the past decade.
Remember that it takes about 17 chemical steps to go from poppy to pill. When she began the work in 2004, Smolke started early in the process and went about halfway through these chemical steps. In a 2008 paper she reported success in that first phase of the project when her bioengineered yeast produced a precursor to thebaine–one of the three principal opiates.
In her new paper, Smolke started with thebaine obtained from poppies, put this into her bioengineered yeast and got refined opioids at the end of the process.
Now her team must extend the 2008 process from sugar to thebaine. Once she forges this missing link in the chain of biochemical synthesis, she will have produced a bioengineered yeast that can perform all 17 steps from sugar to specific opioid drugs in a single vat.
“We are already working on this,” she said.
Smolke said it could take several more years to perfect these last steps in the lab and scale up the process to produce large sized batches of bioengineered opioids that are pharmacologically identical to today’s drugs that start in a field and are refined in factories.
“This will allow us to create a reliable supply of these essential medicines in a way that doesn’t depend on years leading up to good or bad crop yields,” Smolke said. “We’ll have more sustainable, cost-effective, and secure production methods for these important drugs.”
Story Source:
The above story is based on materials provided by Stanford School of Engineering. Note: Materials may be edited for content and length.
Journal Reference:
- Kate Thodey, Stephanie Galanie, Christina D Smolke. A microbial biomanufacturing platform for natural and semisynthetic opioids. Nature Chemical Biology, 2014; DOI: 10.1038/nchembio.1613