Researchers at Columbia University Medical Center (CUMC) have identified the immune cells responsible for destroying hair follicles in people with alopecia areata, a common autoimmune disease that causes hair loss, and have tested an FDA-approved drug that eliminated these immune cells and restored hair growth in a small number of patients.
The results appear in today’s online issue of Nature Medicine.
In the paper, the researchers report initial results from an ongoing clinical trial of the drug, which has produced complete hair regrowth in several patients with moderate-to-severe alopecia areata. Data from three participants appear in the current paper; each patient experienced total hair regrowth within five months of the start of treatment.
“We’ve only begun testing the drug in patients, but if the drug continues to be successful and safe, it will have a dramatic positive impact on the lives of people with this disease,” said Raphael Clynes, MD, PhD, who led the research, along with Angela M. Christiano, PhD, professor in the Departments of Dermatology and of Genetics and Development at CUMC.
Alopecia areata is a common autoimmune disease that causes disfiguring hair loss. The disease can occur at any age and affects men and women equally. Hair is often lost in patches on the scalp, but in some patients it also causes loss of facial and body hair. There are no known treatments that can completely restore hair, and patients with the disease experience significant psychological stress and emotional suffering.
Scientists have known for decades that hair loss in alopecia areata occurs when cells from the immune system surround and attack the base of the hair follicle, causing the hair to fall out and enter a dormant state. Until now, the specific type of cell responsible for the attack had been a mystery. A major clue was uncovered four years ago in Dr. Christiano’s genetic study of more than 1,000 patients with the disease. That study suggested that a “danger signal” in the hair follicles of patients — not previously linked to alopecia areata — attracts the immune cells to the follicle and sparks the attack.
The current paper describes how the team first studied mice with the disease, then tracked backward from the danger signal to identify the specific set of T cells responsible for attacking the hair follicles. Further investigation of mouse and patient cells revealed how the T cells are instructed to attack and identified several key immune pathways that could be targeted by a new class of drugs, known as JAK inhibitors.
Two FDA-approved JAK inhibitors tested separately by the researchers — ruxolitinib and tofacitinib — were able to block these immune pathways and stop the attack on the hair follicles. In mice with extensive hair loss from the disease, both drugs completely restored the animals’ hair within 12 weeks. Each drug’s effect was also long-lasting, as the new hair persisted for several months after stopping treatment.
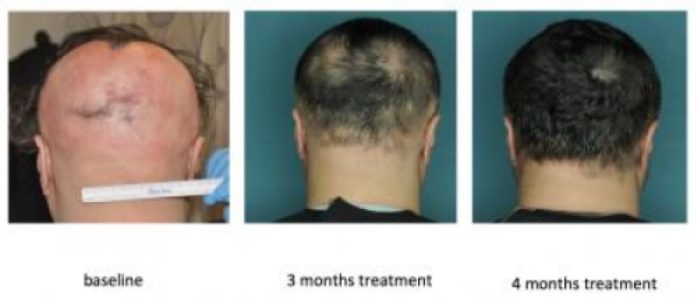
Together with Julian Mackay-Wiggan, MD, MS, director of the Clinical Research Unit in the Department of Dermatology at CUMC and a practicing dermatologist at NewYork-Presbyterian/Columbia who treats patients with multiple types of hair loss, the researchers rapidly initiated a small open-label clinical trial of ruxolitinib — which is FDA approved for the treatment of a blood disorder — in patients with moderate-to-severe alopecia areata (with more than 30 percent hair loss). In three of the trial’s early participants, ruxolitinib completely restored hair growth within four to five months of starting treatment, and the attacking T cells disappeared from the scalp.
“We still need to do more testing to establish that ruxolitinib should be used in alopecia areata, but this is exciting news for patients and their physicians,” Dr. Clynes said. “This disease has been completely understudied — until now, only two small clinical trials evaluating targeted therapies in alopecia areata have been performed, largely because of the lack of mechanistic insight into it.”
“The timeline of moving from genetic findings to positive results in a clinical trial in only four years is astoundingly fast and speaks to this team’s ability to perform translational science of the highest caliber,” said David Bickers, MD, the Carl Truman Nelson Professor of Dermatology and chair of the Department of Dermatology at CUMC and dermatologist-in-chief at NewYork-Presbyterian/Columbia. A practicing dermatologist who cares for many patients with alopecia areata himself, Dr. Bickers said, “There are few tools in the arsenal for the treatment of alopecia areata that have any demonstrated efficacy. This is a major step forward in improving the standard of care for patients suffering from this devastating disease.”
But as Dr. Christiano knows firsthand from her own personal experience with the disease, alopecia areata is too often dismissed as simply an appearance-altering disease. “Nothing could be further from the truth,” she said. “Patients with alopecia areata are suffering profoundly, and these findings mark a significant step forward for them. The team is fully committed to advancing new therapies for patients with a vast unmet need.”
The paper is titled, “Alopecia Areata is Driven by Cytotoxic T Lymphocytes and is Reversed by JAK Inhibition.” The other contributors are Luzhou Xing, Zhenpeng Dai, Ali Jabbari, Jane E. Cerise, Claire Higgins, Weijuan Gong, Annemieke de Jong, Sivan Harel, Gina M. DeStefano, Lisa Rothman, Pallavi Singh, Lynn Petukhova, all at CUMC.
This work was supported in part by USPHS NIH/NIAMS R01AR056016 (to A.M.C.) and R21AR061881 (to A.M.C and R.C.), R01CA164309 and a Shared Instrumentation Grant for the LSR II Flow Cytometer (S10RR027050) to R.C., the Columbia University Skin Disease Research Center (P30AR044535), as well as the Locks of Love Foundation and the Alopecia Areata Initiative. J.E.C. is supported by (T32GM082271) Medical Genetics Training Grant (to A.M.C.). A.J., C.H., S.H., and A.D. are recipients of Career Development Awards from the Dermatology Foundation, and A.J. is also supported by the Louis V.Gerstner, Jr. Scholars Program.
Dr. Clynes worked on this study while an associate professor in the departments of Pathology and Cell Biology, Medicine, and Dermatology at CUMC, and a medical oncologist at NewYork-Presbyterian/Columbia. Dr. Clynes is now employed by Bristol-Myers Squibb, which was not involved in this research.
Columbia University has filed for intellectual property protection on the treatment of alopecia areata with small-molecule JAK inhibitors (PCT/US2011/059029 and PCT/US2013/034688).
Story Source:
The above story is based on materials provided by Columbia University Medical Center. Note: Materials may be edited for content and length.
Journal Reference:
- Luzhou Xing, Zhenpeng Dai, Ali Jabbari, Jane E Cerise, Claire A Higgins, Weijuan Gong, Annemieke de Jong, Sivan Harel, Gina M DeStefano, Lisa Rothman, Pallavi Singh, Lynn Petukhova, Julian Mackay-Wiggan, Angela M Christiano, Raphael Clynes. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nature Medicine, 2014; DOI: 10.1038/nm.3645