For the first time scientists have used cells from a woman with type 1 diabetes to create cloned human embryos from which they extracted embryonic stem cells.
The American-Israeli team also coaxed the stem cells into insulin-producing beta cells, the kind lost in patients with type 1 diabetes.
“These stem cells could therefore be used to generate cells for therapeutic cell replacement,” research leader Dieter Egli, from the New York Stem Cell Foundation Research Institute, said.
Embryonic stem cells are capable of transforming into any tissue in the body, not just insulin-producing cells. Therein lies their medical potential – to regenerate tissue or organs for transplant.
The benefit of stem cells obtained from cloned human embryos is they are genetically.
matched to the person who donated the adult cell, meaning they could be used to personalise therapies for a range of crippling diseases, not just diabetes.
But somatic cell nuclear transfer – the technique used to create Dolly the sheep – is ethically controversial in humans because it involves the creation of embryos for research that are subsequently destroyed.
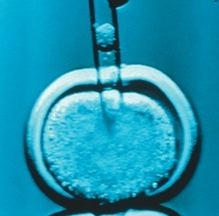
To obtain stem cells from the cloned human embryo, the nucleus of a skin cell from the woman with type 1 diabetes was transferred into a donated human egg, which had had its nucleus removed.
The cells grew into early-stage embryos, or blastocysts, that gave rise to human embryonic stem cells. The researchers published their findings in the journal Nature.
Stem cell research has received a lot of attention lately. Earlier this month Korean and American researchers announced they were the first to use this method to extract stem cells from embryos cloned with adult cells. Previously, only fetal cells had been used to produce cloned embryos. But the breakthrough was overshadowed by controversy surrounding a Japanese scientist accused of falsifying data that supported another kind of groundbreaking stem cell research.
Until cloned embryos were created, researchers had only been successful growing human embryonic stem cells from surplus IVF embryos, which were not a genetic match with the recipient.
Melbourne stem cell researcher Martin Pera said it was a major achievement that stem cells from cloned embryos made with adult cells had been obtained because animal studies had suggested younger cells were better for producing cloned embryos.
“This report shows that the same approach works with adult cells, an important advance because it proves the technique could be used on individual patients to produce perfectly matched tissue for transplantation to treat disease,” Professor Pera, the chairman of stem cell sciences at the University of Melbourne, said.
In a comment piece also published in Nature, an American bioethics associate professor, Insoo Hyun, said while it was easy to see how these cloning studies had broken scientific ground, the justification for this type of research may become a thorny issue in the future.
“It is important to review specifically whether the questions that a custom-made embryo could help to answer justify its creation,” he wrote.
As well as being ethically controversial, stem cells derived from cloned embryos require a large number of donor eggs.
For the New York team to carry out their research, healthy women were paid $8000 each to donate eggs harvested from their ovaries.
Bernie Tuch, the director of NSW Stem Cell Network and CSIRO researcher, said this practice was not allowed in most countries, including Australia.
Creating cloned human embryos for research was legalised in Australia in 2007 and one research group has three licences to conduct this type of research, although they have not been successful obtaining stem cells from cloned embryos.
In most countries, including Australia, it is illegal to create cloned human embryos to implant into a woman.
Source: Sydney Morning Herald